OF Pt-Ba/Al2O3 MONOLITHS FOR DIESEL EXHAUST CONTROL
advertisement
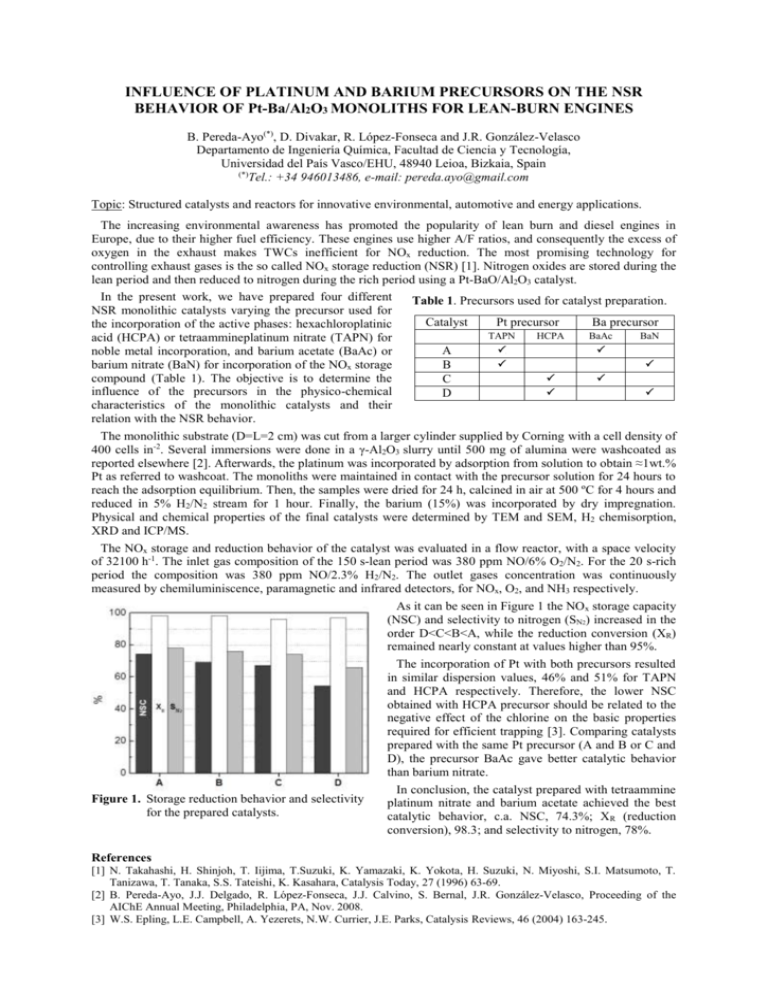
INFLUENCE OF PLATINUM AND BARIUM PRECURSORS ON THE NSR BEHAVIOR OF Pt-Ba/Al2O3 MONOLITHS FOR LEAN-BURN ENGINES B. Pereda-Ayo(*), D. Divakar, R. López-Fonseca and J.R. González-Velasco Departamento de Ingeniería Química, Facultad de Ciencia y Tecnología, Universidad del País Vasco/EHU, 48940 Leioa, Bizkaia, Spain (*) Tel.: +34 946013486, e-mail: pereda.ayo@gmail.com Topic: Structured catalysts and reactors for innovative environmental, automotive and energy applications. The increasing environmental awareness has promoted the popularity of lean burn and diesel engines in Europe, due to their higher fuel efficiency. These engines use higher A/F ratios, and consequently the excess of oxygen in the exhaust makes TWCs inefficient for NOx reduction. The most promising technology for controlling exhaust gases is the so called NOx storage reduction (NSR) [1]. Nitrogen oxides are stored during the lean period and then reduced to nitrogen during the rich period using a Pt-BaO/Al2O3 catalyst. In the present work, we have prepared four different Table 1. Precursors used for catalyst preparation. NSR monolithic catalysts varying the precursor used for Catalyst Pt precursor Ba precursor the incorporation of the active phases: hexachloroplatinic TAPN HCPA BaAc BaN acid (HCPA) or tetraammineplatinum nitrate (TAPN) for A noble metal incorporation, and barium acetate (BaAc) or barium nitrate (BaN) for incorporation of the NOx storage B compound (Table 1). The objective is to determine the C influence of the precursors in the physico-chemical D characteristics of the monolithic catalysts and their relation with the NSR behavior. The monolithic substrate (D=L=2 cm) was cut from a larger cylinder supplied by Corning with a cell density of 400 cells in-2. Several immersions were done in a γ-Al2O3 slurry until 500 mg of alumina were washcoated as reported elsewhere [2]. Afterwards, the platinum was incorporated by adsorption from solution to obtain ≈1wt.% Pt as referred to washcoat. The monoliths were maintained in contact with the precursor solution for 24 hours to reach the adsorption equilibrium. Then, the samples were dried for 24 h, calcined in air at 500 ºC for 4 hours and reduced in 5% H2/N2 stream for 1 hour. Finally, the barium (15%) was incorporated by dry impregnation. Physical and chemical properties of the final catalysts were determined by TEM and SEM, H2 chemisorption, XRD and ICP/MS. The NOx storage and reduction behavior of the catalyst was evaluated in a flow reactor, with a space velocity of 32100 h-1. The inlet gas composition of the 150 s-lean period was 380 ppm NO/6% O2/N2. For the 20 s-rich period the composition was 380 ppm NO/2.3% H2/N2. The outlet gases concentration was continuously measured by chemiluminiscence, paramagnetic and infrared detectors, for NOx, O2, and NH3 respectively. As it can be seen in Figure 1 the NOx storage capacity (NSC) and selectivity to nitrogen (SN2) increased in the order D<C<B<A, while the reduction conversion (XR) remained nearly constant at values higher than 95%. The incorporation of Pt with both precursors resulted in similar dispersion values, 46% and 51% for TAPN and HCPA respectively. Therefore, the lower NSC obtained with HCPA precursor should be related to the negative effect of the chlorine on the basic properties required for efficient trapping [3]. Comparing catalysts prepared with the same Pt precursor (A and B or C and D), the precursor BaAc gave better catalytic behavior than barium nitrate. In conclusion, the catalyst prepared with tetraammine Figure 1. Storage reduction behavior and selectivity platinum nitrate and barium acetate achieved the best for the prepared catalysts. catalytic behavior, c.a. NSC, 74.3%; XR (reduction conversion), 98.3; and selectivity to nitrogen, 78%. References [1] N. Takahashi, H. Shinjoh, T. Iijima, T.Suzuki, K. Yamazaki, K. Yokota, H. Suzuki, N. Miyoshi, S.I. Matsumoto, T. Tanizawa, T. Tanaka, S.S. Tateishi, K. Kasahara, Catalysis Today, 27 (1996) 63-69. [2] B. Pereda-Ayo, J.J. Delgado, R. López-Fonseca, J.J. Calvino, S. Bernal, J.R. González-Velasco, Proceeding of the AIChE Annual Meeting, Philadelphia, PA, Nov. 2008. [3] W.S. Epling, L.E. Campbell, A. Yezerets, N.W. Currier, J.E. Parks, Catalysis Reviews, 46 (2004) 163-245.










