Pulsed Electrical Discharges Assisted Dielectric Pellets/Catalysts for Diesel Engine Exhaust Treatment
advertisement

Rajanikanth and Raui: Pulsed Electrical Discharges for Diesel Engine Exhaust Treatment
616
Pulsed Electrical Discharges Assisted by Dielectric
Pellets/Catalysts for Diesel Engine Exhaust Treatment
B. S. Rajanikanth and V. Ravi
Dept. of High Voltagc Engineering
Indian Institute of Science
Bangalore 560012, India
ABSTRACT
This paper'reports the study on gaseous pollutants (NOx, CO and He's) using a
combination of electric discharge plasma and catalysts. In this study, catalysts being used in conventional catalytic converters of gasoline engine were tried, for the
first time, in diesel engine exhaust treatment assisted by electric discharge plasma.
Initial studies investigate the effect of packed dielectric materials and catalysts on
NOx removal. Both conventional and nun-conventional catalysts were used in the
studies. With plasma alone, the removal efficiency of oxides of nitrogen was around
75% and with a suitable combination of catalytic reactor the removal efficiency was
as high as 90%.Among the catalysts studied, a new catalyst (CuMnAlO,) was found
to be as effective as the conventional expensive catalyst. The formation of byproducts like N,O and HNO, have been studied and the results are discussed.
v
1
INTRODUCTION
HICULAR pollution is a serious problem today.
Due to increased growth in vehicular traffic, the
progress achieved through existing control devices are getting offset. The problem is more serious when it comes to
diesel engines. The problem with the conventional after
treatment technique such as a three-way catalytic converter, which has proved to be very successful in gasoline
engines, cannot be applied to lean burn spark ignition engines or diesel engines because these engines burn their
fuel with a high excess of air and the oxygen in the exhaust gas stream prevents the catalytic decomposition of
oxides of nitrogen. In addition, the precious metals used
in the conventional catalytic converters are highly expensive and originate from limited natural sources. This has
led the researchers to look for alternative measures for
the removal of NOx resulting in the emergence of nonthermal plasma as a technique for gas treatment. Nonthermal plasma associated with electrical discharges is an
excellent technique for the removal of oxides of nitrogen
and is proved to be cost effective [1-51. Non-thermal
plasma alone will not be able to remove all the major pollutants present in the automobile exhaust. However, when
the discharge plasma is combined with catalysts, there is a
scope for removal of these pollutants.
Recently there have been some efforts by researchers in
combining catalysts with electric discharge plasma [6-11]
but none of these studies was performed using actual
Monu.~cnptrereiwd on 5 October 2001, ~n f i n d form 7 May 2002.
diesel engine exhaust. Since actual exhaust consists of
multitude components, the simulated exhaust may provide
an incomplete picture of the processes involved. The reactions taking place during the treatment of actual exhaust
by pulsed discharges vary largely from that of the simulated exhaust. The investigations of a pulsed corona reactor for treating heavy-duty diesel engine emissions by
Grothaus and Fanick [121 resulted in 60% NOx removal
efficiency. Although oxidizing reactions are more pronounced in the case of diesel exhaust (which lead to the
formation of NO, and HNOJ, there was still a marked
decrease in total NOx. The same authors have also studied the effect of combination of pulsed corona discharges
and catalysts and reported NOx removal efficiency of
about 70% [131. They have not made known the type of
catalysts used.
The present work intends to study the feasibility of
combining electric discharge plasma with conventional/
non-conventional catalysts for treating diesel engine exhaust. The diesel engine was operated under no load condition. Though main emphasis was given to the NOx removal, the concentrations of CO and HC's were also monitored simultaneously. Initial studies include the effect of
packed dielectric materials and catalysts in a plasma reactor on NOx removal. Studies were conducted at different
temperatures. The formation of main byproducts associated with NOx removal is also discussed briefly.
2
EXPERIMENTAL SETUP
Figure 1 shows the experimental setup. The pulse source
consists of a single-phase ac supply, Cockraft-Walton dc
1070-9878/1/$17.00 02002 IEEE
ZEEE Trnnsnctions on Dielectrics and Electrical Insukztion
Vol. 9, No. 4; August 2002
617
h
U
-
Cn=c*R-t.,
~ u lR..M
.
mi
V&*
figure
w pdsr
1. Experimental set up.
voltage multiplier circuit and a rotary spark gap (RSG).
The hemispherical rotating electrode of the RSG was connected to a motor through an insulating rod. By changing
the speed of the motor, the frequency of the pulses applied to the reactor can be controlled. Throughout the experiments the frequency of the pulses was kept constant
at 100 pps (pulses per second). The pulse voltage applied
to the reactor was measured by means of a 150 MHz digital oscilloscope (DL1540, 200MS/s' Yokogawa) connected
through a 20001 voltage divider (EP-SOK, 50 MHz, Peec,
Japan). The power was measured directly from the oscilloscope as the product of voltage and current waveforms.
The specific energy density in Joules per liter was then
calculated as the. ratio of power (W) to the flow rate (liter
per minute). A 6 kW diesel engine, under no load conditions, was used for the experiments. A,part of the exhaust
gas was made to pass through a particulate filter first
(PROGARDTM, Millipore India Ltd.) to filter out solid
particulates down to 4 microns in diameter and then fed
to the reactors. A flow meter connected after the particulate filter was adjusted to get a constant gas flow rate of
0.75 liter per minute (Ipm). The measurement of NOx from
the diesel exhaust was done using an FTIR gas analyzer
(DX-4010, Temet Instruments, Finland). Before feeding
the exhaust gas into the analyzer, the gas was made to
pass through a micro-dust filter to filter out particulates
down to 2 pm. The GASMETTMuses the CALCMETTM
software to compute the concentrations of the components present in the sample gas from the absorbance spectrum.
During the course of experiments, two different reactors were used, specifically the plasma reactor (PR) and
the catalytic reactor (CR). The PR is a dielectric barrier
discharge reactor made up of quartz glass tube of diameter (inner diameter = 15 mm and outer diameter = 17
mni). It consists of a stainless steel wire of 0.1 mm diameter as the inner electrode and aluminum foil wrapped over
the quartz glass tube as the outer electrode. The studies
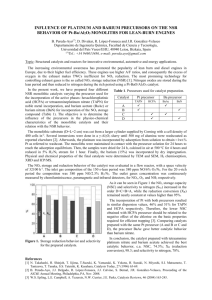
were conducted by filling the PR successively with dielectric pellets and then with catalysts. Figure 2a shows plasma
reactor without pellets and Figure 2b shows the PR filled
\
Glass woo1
(c)
Quartz tube
\
Honeycomb struelure
I
(4
Figure 2. a, reactor without pellets; b, reactor filled with
dielectric/catalyst pellets; c, reactor filled with catalyst pellets; d, reactor filled with honeycomb catalyst.
with dielectric pellets/catalysts. In the present experiments dielectric pellets such as BaTiO, ( e r = 3200), AlzO3
(E, = 10) and glass beads (e, = 10) were used. The experiments were then conducted by replacing the dielectric
pellets with catalysts such as Pd/AI,O,, CuO and Fe,O,.
The catalytic reactors consisted of a pellet bed reactor
and a honeycomb reactor. Figure 2c shows the catalytic
reactor (glass tube of 15 mm diameter and 300 mm length)
filled with catalyst pellets such as Pd/AI,O, and Fe,O,.
The catalysts are packed inside the reactor for a length of
about 70 mm and both the ends were held by glass wool.
Figure 2d shows the catalytic reactor filled with a honeycomb structure coated with a conventional catalyst (PtRh/Ce0,-Al,03) and a non-conventional catalyst
(CuMnAlO,). The honeycomb for the former is of length
120 mm and diameter 25 mm and was inserted in a glass
tube (30 mm diameter, 300 mm long), while for the latter
the honeycomb is of length 120 mm and diameter 12 mm
and was inserted in a glass tube (15 mm diameter, 300 mm
long). In both cases the CR was kept inside an electric
Rajanikanth and Ravi: Pulsed Elechical Discharges for Diesel Engine Exhaust Treatment
618
furnace. A brief explanation of the catalysts used in the
experiments is given below.
1. Pd/Al,O, (palladium coated over alumina). This is
the most commonly used ,conventional two-way oxidation
catalyst for exhaust gas treatment. This was used in the
form of pellets (5mm long and 3mm diameter). The catalyst is active at s 200°C.
2. CuO-ZnO-Al,O, (copper oxide coated over a support of zinc oxide and alumina). This is a base metal catalyst. Before the advent of noble metal catalysts these catalysts were initially used to treat the exhaust gas emissions.
Pellets with size of 4 mm long and 6 mm diameter were
used in the present studies. This is a good oxidation catalyst for the oxidation reactions of CO and HCs. The catalyst is active in a narrow temperature window of
200-300°C.
3. Fe,O,-Cr,O, (Iron oxide doped with chromia). This
is also a base metal catalyst that was used in the treatment of exhaust gas before the advent of noble metal catalysts. Pellets with 4 mm long and 6 mm diameter were
used in the present studies. The oxidation reactions start
around 200°C with this catalyst and the catalyst loses its
activity above 500°C.
4. Pt-Rh/CeO,-Al,O, (platinum and rhodium coated
over a support of ceria and alumina). This is the most
commonly used conventional three-way catalyst for the
auto exhaust treatment. This catalyst is coated on a
cordierite honeycomb structure of length 120 mm and diameter 25 mm. The catalyst is active at > 200°C.
5. CuMnAlO,. This is a new catalyst prepared in the
laboratory. It is a non-conventional catalyst, coated on a
cordierite honeycomb structure of length 120 mm and diameter 12 mm. This is a vely good oxidation catalyst and
works at > 175°C.
3 RESULTS AND DISCUSSION
A diesel engine of 6 kW under no load conditions was
setup in the laboratory. The experiments were conducted
using a filtered exhaust gas. Only a portion of the filtered
exhaust gas from the diesel engine was passed through the
reactors. Before treating the exhaust gas, either with the
PR and/or the CR, the concentrations of CO, THC, NOx,
N,O, H,O, and CO, were measured using a FTIR multicomponent gas analyzer. Also, the concentration of oxy-
Table 1. Initial conccntrations of diesel engine exhaust gas components.
Component
Concentration
co
THC
700 ppm
90 vvm
gen present in the diesel exhaust was measured using a
NDIR gas analyzer. Table 1 shows the initial concentrations of these components present in the diesel engine exhaust gas. In the table, “N0x”means the sum of NO and
NO, concentrations. The concentrations of NO and NO,
were measured individually and then added to get the NOx
concentration. The total hydrocarbons ( T H O are the sum
of various HC‘s present in the exhaust. THC in the present study includes methane (CH,), ethylene (C,H4),
acetylene (C,H,), butane (C,H,”), propene (C,H,), benzene (C,H,), and toluene (C,H,). The experiments were
conducted for three arrangements of the plasma reactors
as shown below.
Case (I)
Gasin
r]
~
Gas out
PACR refers to plasma-assisted catalytic reactor. In
each of the above cases, the relevant exhaust gas component concentrations were measured before and after the
plasma/catalytic treatment. In all the experiments, the PR
was kept at room temperature and the temperature of the
CR alone was varied from room temperature to 300°C.
3.1
STUDIES USING PLASMA REACTOR
(PR)
The effect of dielectric pellets on NOx removal has been
investigated 114,151 and it was found that the efficiency of
the plasma reactor increased by packing it with dielectric
pellets. When the plasma reactor was filled with dielectric
pellets and pulsed high voltage was applied across the pellet bed, the pellets become polarized and an intense electric field is formed around the contact points of each pellet, causing partial discharges between the pellets. This
partial discharge together with the discharge from the wire
(referred to as combined discharge) helps in increasing the
radical production thereby enhancing the chemical reaction rates leading to more NO removal. However, none of
these investigations was conducted on actual diesel engine
exhaust. Experiments on NOx removal using dielectric
pellets have been conducted to verify the above fact using
actual diesel engine exhaust. In these studies the exhaust
was treated using a plasma reactor packed with different
dielectric materials (BaTiO,, Al,O, and glass beads). Experiments were also conducted without using any pellets.
The study is intended to determine the relative performance of various dielectric pellets. All the experiments
were conducted at room temperature. Figure 3a shows the
NOx removal efficiency using PR packed with dielectric
pellets and PR without pellets as function of specific en-
IEEE Transactions on Dielectrics and Electrical Insulation
-m- No plIe1s
I
i
ii
i
i
i
/
b
2x
I
/
i
20
oJ
,
5
,
,
10
,
,
15
,
,
,
20
,
25
,
I
, .
30
I5
40
45
Specific Energy Density (Jn)
(a)
ten
L3PR (no pellets)
I40
-
120
B (W
-z
C
0
.-
110
5
SO
c
u
8
40
20
0
NO
NO2
NOX
N20
(b)
(4
Figure 3. a, NOx removal efficiency for PR packed with and without dielectric pellets as a function of specific energy density; b, comparison of concentrations of NO, NO,, NOx and N,O far PR packed
with BaTiO, and PR without pellets; c, comparison of concentrations of CO and THC for PR packed with BaTiO, and PR without
pellets.
ergy density. Both BaTiO, packed PR and PR without
pellets show maximum NOx removal efficiency. PR packed
with AI,O, and glass beads exhibited high NO removal
but not NOx removal. Also, it should be noted that the
PR without pellets exhibited the least energy consump-
Vol. 9, No. 4; August 2002
619
lion. Figure 3b compares the concentrations of NO, NO,,
NOx and N,O for PR packed with BaTiO, and PR without pellets, before and after treatment, at the maximum
specific energy density. It is observed that the reduction in
NOx is almost the same for both cases. With BaTiO,
packed PR, the NO, production is higher compared to
PR without pellets, which indicates that BaTiO, generates maximum quantity of oxygen species. Consequently,
BaTiO, packed PR generated maximum amount of N,O
as compared to PR without pellets. At maximum specific
energy density (24 J/I) the concentration of N,O increased to 18 ppm from an initial value of 2.5 ppm for
BaTiO, packed PR. For PR without pellets the concentration of N,O was increased to 8 ppm from an initial
value of 2.5 ppm. Figure 3c compares the concentrations
of CO and THC for BaTiO, packed PR and PR without
pellets before and after treatment at the maximum specific energy density. For BdTiO, packed PR the concentration of CO was found to increase from the initial vahe.
At maximum specific energy density, the CO concentration was increased to 725 ppm from an initial value of 700
ppm whereas for PR without pellets there was no change
in initial CO concentration. There is a slight reduction
(10%) in the concentration of THC using BaTiO, packed
PR and PR without pellets. From the above discussion, it
is observed that PR without pellets is a better choice compared to PR filled with dielectric pellets. A detailed study
of NOx removal using PR without pellets is explained in
the following paragraph.
Figure 4a shows the removal efficiencies of NO and NOx
for PR without pellets. When the specific energy density
is around 5 J/1, the concentration of NO was decreased to
45 ppm from an initial value of 115 ppm. This reduction
corresponds to about 60% removal of NO. This is because
of strong oxidation reaction in high oxygen atmosphere
leading to the formation of NO,. The concentration of
NO, was increased to 70 ppm from an initial value of 35
ppm at this specific energy density. So, there is a decrease
in NOx concentration by 34 ppm (from initial 150 ppm to
116 ppm). But up to specific energy density of 13 J/I, the
oxidation reactions were dominant resulting in NO removal efficiency of nearly 80% and NOx removal efficiency of only around 35%. However, beyond 20 J/I, the
concentration of NO, started decreasing thereby increasing the removal efficiency of NOx. The reason for NO,
drop is that the NO, reacts with hydrocarbons and water
to release various other HC by-products, HNO,, N,O etc.
The rate coefficients for these reactions are more favorable at higher radical energies than that at lower radical
energies. At 24 J/I the NOx removal efficiency became
76%. This is well represented in Figure 4b, which shows
the concentrations of NO, NO,, NOx and N,O as a function of specific energy density. It has been reported that
without any additives the reduction of NO into N, in high
oxygen atmosphere is not possible [14,171. In the present
studies, since an actual diesel exhaust was treated, it is
Rajanikanth and Ravi: Pulsed Electrical Discharges for Diesel Engine Exhaust Treatment
620
I
,
5
,
,
.
10
,
15
1
,
20
25
Specific Energy Density (Jn)
(a)
.-.-.
5
.--
10
15
20
25
Specific Energy Density (Jn)
(b)
Figure 4. a, NO and NOx removal efficiencies for PR without peilets; b, concentrations of NO, NO,, NOx and N,O as function of
specific energy density far PR without pellets.
possible that the presence of HCs in the exhaust has played
the role of additives in the decrease of NO concentration
[12]. To correlate this we observed decrease in the concentration of hydrocarbons. At the same time the NOx
removal was also associated with the formation of HNO,
and N,O. The concentration of N,O was found to increase from initial 2.5 ppm to 8 ppm at the maximum specific energy density (24 J/I), Though HNO, was not measured quantitatively, its presence was confirmed by qualitative analysis. For this, the treated exhaust gas was made
to pass through distilled water for about 10 minutes and
this water was tested for its acidity using a pH meter. The
results confirmed the presence of HNO,. The formation
of HNO, is desirable as it could easily be neutralized to a
salt by simple chemical methods.
3.2
STUDIES USING PLASMA-ASSISTED
CATALYTIC REACTOR (PACR)
The efficiency of NO removal achieved by a catalyst assisted with electric discharge plasma at room temperature
has been reported [15] and found to be quite comparable
with that achieved by conventional catalytic converters operated at ~ 3 0 0 ° C .However, the above study was not
conducted by using actual diesel exhaust gas. Also, NOx
removal was not discussed. Hence, the focus of this paper
is placed on the NOx removal by catalysts assisted by electrical discharges using actual diesel engine exhaust gases
and comparison of performances of conventional and
non-conventional catalysts assisted by electrical discharges. The experiments were conducted by packing the
catalysts (in pellet form) in a plasma reactor at room temperature using actual diesel engine exhaust. A reactor
configured in this manner is referred to as a plasmaassisted catalytic reactor (PACR). The studies were limited to room temperature because of the practical difficulties. The catalysts used in the study were Pd/AI,O,, (a
conventional catalyst) and Fe,O, and CuO (both nonconventional catalysts). Figure Sa shows the NOx removal
efficiencies as function of specific energy density for PACR
packed with different catalysts. It is observed that the NOx
removal efficiency is above 60% for low specific energy
densities in all cases. The NOx removal is dominated by
catalytic adsorption rather than by pulsed discharges in
these cases. Fe,O, and CuO showed vely high adsorption
characteristics when compared to Pd/AI,O, catalysts. The
NOx concentration was decreased from 150 ppm to 105
ppm with Pd/AI,O, catalysts. This amounts to 30% removal of NOx. However, when the specific energy density
is around 5 J/I the NOx removal efficiency became 63%.
For higher specific energy densities, the removal was linear and reached a maximum of 88%. With the CuO packed
PACR, the NOx removal efficiency was almost saturated
beyond a specific energy density of 5 J/1. CuO alone adsorbed nearly 70 ppm NOx out of initial 150 ppm. The
maximum NOx removal efficiency obtained with the CuO
packed PACR was 94%. For the Fe,O, packed PACR,
the NOx removal efficiency was 90% for a specific energy
density of only 5 J/I. This is because Fe,O, catalysts adsorbed 116 ppm of NOx (77% adsorption). Beyond this
specific energy density, there was no significant increase
in removal efficiency. Figure Sb compares the concentrations of NO, NO,, NOx and N,O before and after treatment for PACR packed with catalyst pellets. Though the
NOx removal was higher for CuO and Fe,O, packed
PACR, the N,O production was higher for these two when
compared to Pd/AI,O, catalysts.
Figure 5c compares the concentrations of CO and THC
before and after treatment for PACR packed with catalyst
pellets at maximum specific energy density (24 J/I). The
Fe,O, packed PACR showed maximum removal of CO
followed by the Pd/AI,O, and CuO cases. The readings
were taken by passing the exhaust gas through the catalyst
until steady readings were obtained. Once the readings
were stabilized L e . no more adsorption is possible by the
catalysts) the discharges were produced inside the reactor
by applying the pulses. It was found that the presence of
I
IEEE Transactions on Dielectrics and Electrical Insulation
Val. 9, No. 4; August 2002
62 I
Table 2. CO and THC removal efficiencics using different catalysts
for PACR at 2PC.
100,
Removal Efficiencies (%)
I
sol
,
5
,
,
-U-
PdlA120,
-0-
cuo
-X-
Fa20,
,
io
7s
20
I
2s
Specific Energy Density (Jn)
(a)
180
-
140
IIIII]PACR (CuO)
120
E
80
e
E
8
d
.
60
8
40
0
Pd/AI,.0i,
CO
THC
18
I
26
CUO
11
42
.,
Fe-0,
22
19
moved by the catalysts in presence of pulsed discharges.
There was about 50 ppm of CO adsorption using
Pd/AI,O, catalysts. The PACR packed with Pd/AI,O,
catalysts removed 125 ppm of CO. So, there was a decrease in CO concentration by 75 ppm in the presence of
pulsed discharges. Adsorption of CO by CuO was much
less (10 ppm). However, the presence of discharges assisted the catalysts in removing 80 ppm of CO. Maximum
removal of THC was achieved by CuO packed PACR followed by Fe,O, and Pd/AI,O, packed PACR. In this case
too, the pulsed discharges assisted the catalysts in removing the hydrocarbons.
The above results show that even at room temperature
some reactions were taking place on the surface of the
catalyst in presence of pulsed discharges, which resulted
in the removal of NOx, CO and THC. Though the removal efficiencies of CO and THC are low in these studies (Table 21, it is still quite interesting considering the
fact that the catalysts are not active at room temperature.
100
E
.e
c
Pollutants
20
0
NO
NO2
NZO
NOX
...
E
-a
6W
n
E
f5
5
IW
2w
0
co
THC
(c)
Figure 5. a, NOx removal cfficiency for PACR packed with catalyst
pellcts; b, comparison of concentrations of NO, NO,, NOx and N 2 0
for PACR packed with catalyst pellcts; c, comparison of concentrations of CO and THC for PACR packed with catalyst pellcts.
discharges enhanced the CO removal by catalyst. The c&
alyst Fe,O, alone removed 125 ppm of CO (by adsorption
process) and the combination of discharges and catalyst
removed 155 ppm of CO. Hence 30 ppm of CO was re-
3.3 STUDIES USING A COMBINATION
OF PLASMA/CATALYTIC REACTORS
In this study, the exhaust gas from the diesel engine is
made to pass through a combination of catalytic and
plasma reactors connected in series. This is an attempt to
study the feasibility of placing a plasma reactor along the
exhaust channel in a vehicle without disturbing the existing catalytic converters. Both conventional and non-conventional catalysts were used in the form of pellets as well
as a honeycomb structure. Initial studies were focused on
selecting a suitable combination of PR and CR. For this,
catalytic reactor packed with Pd/AI,O, pellets was tested
for two combinations with PR. In the first case, CR was
put after PR (this combination is referred as PR+CR)
and in the next case CR was put before PR (this combination is referred as CR+PR). The studies were carried out
at different temperatures of CR. It should be noted that
in all these experiments the PR was kept at room temperature and only the temperature of the CR was raised. Preliminary results in regard to this were presented in [16].
Fig. 6a shows the NOx removal efficiencies for P R + C R
combination at different temperatures. At room temperature and at lOO"C, the catalytic adsorption is high resulting in the high NOx removal efficiencies. The NOx removal efficiency decreases with increase in the temperature of the catalytic reactor. The NOx removal efficiency
was as low as 35% when the temperature of CR is raised
Rajanikanth and Raui: Puked Electrical Discharges for Diesel Engine Exhaust Treahnenl
622
-
-
-.-
27 'C
-.-
.
P
80-
5
0
.
5
._
-
-IPR
+ CR at 200'C
Io
- -0- PR + CR at 300%
.
CR + PR at ZOO'C
1
CR + PR at 300'C
-X-
70-
so:
5040:
L
.
-0- 100%
-x- 200 'C
/0
300 'C
0
5
4s
10
20
21
Specific Energy Density (Jfl)
5
16
IO
20
25
Specific Energy Density (Jfl)
(a)
(a)
-.-NO
4 9"
20
o\o--3
0
0
0
5
10
.
45
20
25
Speclnc Energy Density (Jll)
(b)
Figure 6. a, NOx removal efficiencies for PR+CR (Pd/AI,O,)
combination at different temperatures; b, concentrations of NO, NO,
and NOx for PR+CR (Pd/',OJ
combination at 200°C.
to 300°C. This was because the NO, generated by PR was
adsorbed on the catalyst surface initially and slowly dissociated back into NO. This was evident as high concentration of NO was still left after the treatment with P R + C R
combination. Figure 6b shows the concentrations of NO,
NO, and NOx as function of specific energy density at
200°C with PR CR combination. It is observed that the
concentration of NO, was very low indicating the adsorption by catalyst whereas that of NO was high. This clearly
showed that some of the NO, adsorbed on the catalyst
was slowly dissociated into NO.
+
For C R + P R combination, it is found that at all temperatures the NOx removal efficiency is vely high when
compared to that of P R + C R combination. For example,
Fig. 7a compares the removal efficiencies of NOx, for both
the combinations, at 200°C and at 300°C. At 200"C, NOx
removal efficiency for CR PR combination was about
40% higher than that of P R + C R combination and at
+
@)
Figure 7. a, comparison of NOx removal efficiencies of PR+CR
and CR+PRcombinations(catalyst-Pd/Al,O,)
at 200°C and 300°C;
b, comparison of concentrations of CO and THC before and after
treatment for both P R + C R and C R + P R combinations at 300°C.
300"C, the difference was about 50%. The N,O concentrations were monitored for both the combinations. There
was only negligible increase in the concentration of N,O
in both the combinations.
Figure% compares the concentrations of CO and THC
before and after treatment for both the combinations at
300°C. The performance of both the combinations was
equally good with respect to CO and THC removal and
there is only a negligible difference between the two.
It should be noted here that the above set of experiments were conducted with only one catalyst (Pd/AI,OJ
in the CR but for two combinations, CR PR and PR +
CR. It is inferred that the CR + PR combination performs
better in NOx removal and henceforth the studies were
reported for C R + P R combination, The advantages of using C R + P R combination include preventing the deposition of HNO, formed by PR on the catalysts (as observed
+
IEEE Transactions on Dielectrics and Electrical Insulation
$00
-
110
-
e
E
I
Vol. 9, No. 4; August 2002
w
623
110
70
A
60 -
40
-
20 -
51105115 Enemy D c n r I r 2 4 Jn
0
X
w
PdlAl,O,
Fe20,
PtdhlCeO,-Al,O,
CuMnAIO,
-.-
-0-
0 7
10
13
10
3.
20
PdlAIzO
Fe,O,
25
Specific Energy Density (JII)
Temperature ('CI
Figure 8. NOx removal efficiency for C R + P R combination using
different catalysts as a function of temperature.
(a)
under P R t C R case) and achieving maximum NOx removal efficiency for a given temperature of the CR. Further experiments were carried out using the C R + P R
combination.
Figure 8 shows the NOx removal efficiencies as a function of temperature of CR for CR + PR combination employing all the four catalysts. It can be observed that as
temperature increases the removal efficiency decreases. At
room temperature the NOx removal was 96% and at
300"C, it was 83% for Pd/AI,O, catalysts. This is because
at room temperature the catalytic adsorption aids the NOx
removal. At 300"C, most of the NO was oxidized to NO,
and reduction in total NOx concentration was reduced.
However, this decrease is not very high and NOx removal
efficiency is always above 80%. Hence, NOx can be removed effectively irrespective of the temperature of the
catalytic reactor. All the catalysts showed maximum removal of CO and THC at 200°C and 300°C. This is hecause the catalysts become active only above a temperature of 200°C.
1100
EP
,g 400
?6
STUDIES WITH CR+PR COMBINATION
(CR CONSISTS OF CATALYSTS IN THE FORM
OF PELLETS)
3.3.1
g
200
0
Two kinds of catalysts were used here, Pd/Al,O, (conventional) and Fe,O, (non-conventional) in the pellet
form. The performances of both the catalysts were studied
in combination with PR. Figure 9a shows the NOx removal efficiencies.achieved using these catalysts in CR
PR combination when CR is maintained at 300°C. The
catalysts alone did not remove much NOx. For example,
Pd/AI,O, could remove only 35 ppm of NOx (about 20%)
and Fe,O, could remove only 15 ppm of NOx (about 10%)
even at 300°C in the absence of discharge plasma. This is
because at high oxygen atmosphere like diesel exhausts
the catalysts oxidize most of the NO into NO, keeping
NOx constant. But when CR is combined with PR, the
+
0
co
THC
(4
Figure 9. a, NOx removal efficiencies for C R + P R combination at
300°C (catalysts in the form of pellets); b, comparison of concentrations of NO, NO, and NOx before and after treatment for C R + P R
combination at 300°C (catalysts in the form of pellets); c, comparison
of concentrations of CO and THC before and after treatment for
C R + P R combination at 300°C (catalysts in the form of pellets).
NOx removal efficiencies increased to 83%. Here the NOx
removal was because of the presence of PR, the processes
of which have already been explained. With Fe,O, pellets
624
Rajanikanth and Rani: Puked Elechical Dischargesfor DieseI Engine Exhaust Treatment
inside the CR the NOx removal efficiency was slightly
higher than that with Pd/AI,O,. However, at lower specific energy densities Pd/AI,O, showed better performance. The N,O production was very low with both the
catalysts. Figure Yb shows the concentrations of NO, NO,
and NOx before and after treatment using Pd/Al,O, and
Fe,O, catalysts in C R + P R combination when CR is
maintained at 300°C.
Figure Yc shows the concentrations of CO and THC before and after treatment using Pd/Al,O, and Fe,O, catalysts in CR + PR combination when CR is maintained at
300°C. With catalysts alone, CO was almost completely
removed for Pd/AI,O, hut when PR is placed after CR
the CO concentration was slightly increased. This is because of CO, being reduced to CO and 0, in presence of
pulsed discharges in the PR. It is also true that partial
oxidation of H C species will result in CO production.
However, this increase is very negligible (15-20 ppm) and
since diesel engines emit lesser CO concentration this increase should not he a problem. The same is observed
with Fe,O, catalyst also. Both the catalysts were found to
he equally good in removing CO and THC when combined with a plasma reactor. It is inferred from these experiments that the catalyst Fe,O, was found to be as good
as conventional catalyst Pd/AI,O, in the removal of CO
and THC.
3.3.2 STUDIES WITH CR + PR COMBINATION
(CR CONSISTS OF CATALYSTS IN
HONEYCOMB STRUCTURE)
Current catalytic converters employ catalysts coated on
a honeycomb structure for treating auto exhausts. We have
conducted experiments with a section of one such honeycomb structure (Bharat Heavy Electricals Limited, India
make) and coating the same with a conventional as well as
a non-conventional catalyst. The honeycomb structure was
then placed inside a glass tube forming a catalytic reactor.
The plasma reactor was placed after this catalytic reactor
and the details of the studies are as reported below. A
commonly used conventional catalyst such as PtRh/CeO,-Al,O, and a new, laboratory made non-conventional catalyst CuMnAlO, were used for coating on the
cordierite honeycomb structure. Fig. 10a shows the NOx
removal efficiencies with these catalysts in C R + P R combination when CR is maintained at 300°C. The catalysts
alone could not remove significant amount of NOx. Both
the catalysts removed only 30 ppm of NOx out of total 150
ppm in the absence of pulsed discharges, which amounts
to 20% of NOx removal. However, when PR is placed after CR more than 85% of the NOx was removed in the
combined process with both the catalysts. Fig. 10b shows
the concentrations of NO, NO, and NOx before and after
treatment for C R + P R combination when CR is maintained at 300°C. The N,O production was low in both the
cases.
Figure 10c shows the concentrations of CO and THC
before and after treatment using these two catalysts in CR
+PR combination when CR is maintained at 300°C. Catalysts alone oxidized CO to CO, to a maximum extent
(nearly 100%) and the increase in CO (due to dissociation
of CO,) concentration because of PR was negligible. THC
was also removed effectively by both the catalysts. It is
observed from the above set of experiments that the new,
laboratory made catalyst CuMnAlO, performed equally
effective when compared to the conventional expensive
catalyst. The new catalyst is also inexpensive though the
exact cost per gram is yet to he evaluated. This laboratory
made catalyst appears to he a promising alternative to the
conventional catalyst.
3.4 RELATIVE PERFORMANCE OF THE
REACTORS USED IN CURRENT STUDY
In this section, the results obtained by using different
reactors are compared. In each kind of reactor, the best
one was selected. Since not all the reactors were studied
under different temperature conditions, only room temperature studies arc compared. In the first reactor i.e. PR,
PR without pellets was found to he better as compared to
PR packed with dielectric pellets. In the second kind of
reactor i.e. PACR, Fe,O, catalyst showed better performance than CuO and Fe,O,. In the third reactor configuration, CR. PR, Pd/AI,O, (pellets) and CuMnAlO,
(coated on honeycomb structure) catalysts were proved to
he better. All the above reactors were selected by taking
into account the removal efficiencies of NOx, CO and
THC. Fig. l l a shows NOx removal efficiencies for all these
reactors at room temperature. PACR packed with Fe,O,
and C R + P R employing Pd/AI,O, catalyst showed m a i mum removal efficiencies even at lower specific energy
densities. This is because of high adsorption characteristic
nature of these catalyst pellets at room temperature.
CuMnAlO, also exhibited adsorption characteristics at
room tcmperature hut it was not as high as that of Fe,O,
as the catalyst is coated onto a honeycomb structure. The
NOx removal efficiency was 76% for PR without pellets
and is less than that of the other reactors at room temperature.
+
Figure l l b shows the concentrations of CO and THC
before and after treatment for all the reactors at room
temperature. There was no change in initial concentration
of CO for PR without pellets and CR (CuMnAlO,)+PR
reactors after the treatment. PACR packed with Fe,O,
could remove about 150 ppm of CO at room temperature
itself. This is an important result as catalysts seldom work
at room temperatures. With the reactor CR (Pd/AI,O,)+
PR, there was a small increase in the CO (10 ppm) concentration from initial concentration. Though the catalyst
adsorbed about 10 ppm of CO the PR placed after CR
reduced CO, into CO. However, the increase is very small
and can he ignored. The removal of THC is more with
IEEE Transactions on Dielectrics and Electrical Insulution
Vol. 9, No. 4; August 2002
' 090
°
625
I-
+--'
20-1
,
,
,
v
1
,
13
10
6
0
X
20
3
25
13
40
PACR (Fe,O,I
CR IPdIA1,OJ
+ PR
CR ICuMnAlOJ + PR
10
15
Specific Energy Density (Jll)
Specific Energy Density (JII)
(a)
(a)
800
-I
so0
n
2
L
IO0
E
I
C
8
g
200
0
0
co
THC
(b)
. .
Figure 11. a, NOx rcmovai cfiiciencies for different reactors at room
temperaturc as B function of specific energy density; b, concentrations of CO and THC before and after treatment for different reactors at room temperature.
PACR packed with Fe,O, and other reactors showed
moderate performance. PR without pellets showed the
least removal of THC.
4
CONCLUSIONS
H E major conclusions drawn from the present studies
Tare,
co
THC
(C)
Figure 10. a, NOx removal efficiencies for C R + P R combination at
300°C (catalysts in the form of honeycomb); h, comparison of cancentrations of NO, NO, and NOx before and after treatment far C R +
PR combination at 300°C (catalysts in the form of honeycomb); c,
campariron of concentrations of CO and THC before and after
treatment for C R + P R combination at 300°C (catalysts in the form of
honeycomb).
1. The room temperature studies of plasma reactor (PR)
packed with and without dielectric pellets showed that the
NOx removal efficiency is higher for PR without pellets.
Furthermore, when the exhaust gas was treated with a PR
packed with BaTiO, pellets, an increase in CO was observed as well as the formation of higher levels of N,O.
2. The performance of plasma-assisted catalytic reactor
(PACR), where plasma and catalyst are in the same reactor, showed that even at room temperature some reactions were taking place on the surface of the catalyst in
presence of pulsed discharges resulting in the removal of
626
Rajanikanth and Raui: Pulsed Electrical Dischargesfor Diesel Engine Exhaust Treatmenl
NOx, CO and THC. Though the removal efficiencies of
CO and THC are not high in these studies, it is still quite
interesting considering the fact that the catalysts are not
active at room temperature.
3. C R + P R combination was found to be better than
PR CR combination. For CR + PR combination the NOx
removal efficiency was always high irrespective of the
temperature of the CR.
+
4. In the absence of plasma the catalyst could remove
NOx only up to about 15-20% even at 300°C. However, in
presence of plasma the NOx removal efficiency was always higher than 80% for temperatures up to 300°C.
5. The studies conducted using a new, lab-made, less
expensive catalyst (CuMnAlO,) used in conjunction with
pulsed discharge showed promising results. Interestingly,
the new catalyst performed equally well in the removal of
NOx, CO and THC from the diesel engine exhaust when
compared to that with conventional three-way catalyst.
ACKNOWLEDGMENTS
We thank the Department of Solid State and Structural
Chemistty Unit, 11% and Bharat Heavy Electricals Limited, Bangalore, for providing some of the catalysts during
the course of our experiments.
REFERENCES
111 S. Masuda, and H. Nakao, “Control of NOx by Positive and
Negative pulsed Corona Discharges”, IEEE Trans. IA, Vol. 26,
pp. 374-383, 1990.
I21 G. Dinelli, L. Civitano. and M. Rea, “Industrial Experiments on
Pulse Corona Simultaneous Removal of NOx and SO, from Rue
Gas”, IEEE Trans. IA, Val. 26, pp. 535-541, 1990.
131 J. S . Chang, P. A. Lawless, and T. Yamamoto, “Corona Discharge Processes”, IEEE Trans. Plasma Science, Vol. 19, No. 6,
pp. 1152-1166, 1991.
141 G. E. Vogtlin, and B. M. Pcnetrante, “Pulsed Corona Discharge
for Removal of NOx from Flue Gas”, NATO AS1 Series 34. Oxford, U.K Springer-Verlag, 1993, Part.B, pp. 187-198.
151. Y. S . Mok and 1. S. Nam. “Positive Pulscd Corona Discharee
.
Process for Simultaneous Removal of SO, and NOx from IronOre Sintering Flue Gas”, IEEE Trans. Plasma Science, Vol. 27,
pp. 1188-1196, 1999.
[61 T. Oda, T. &to. T. Takahashi. and K. Shimizu. “Nitric Oxide
Decompositron in Air by Using Non-thermal Plasma Processrng
~~
~
1~
With Additives and Catalyst”, IEEE IAS Annual Meeting. pp.
1803-1807, 1996.
171 K. Shimizu and T. Oda, “DeNOx Process in Flue Gas Combined With Nan-thermal Plasma and Catalyst”, IEEE-IAS Annual Mccting, pp. 1942-1949, 1997.
[El K. Shimizu, T. Hiram, and T. Oda, “Effect of Water Vapor and
Hydrocarbons in Removing NOx by Using Nonthermal Plasma
and Catalyst”, IEEE Trans. IA, Vol. 37, pp. 464-471, 2001.
191 A. Mizuno, K. Shimizu, K. Yanagihara, K. Kinoshita, K. Tsunoda, H. H. Kim, and S. Katsura, “Effect of Additives and Catalysts on Removal of Nitrogen Oxides Using Pulsed Discharge”,
IEEE IAS Annual Meeting, pp. 1806-1812, 1996.
[lo] H. H. Kim, K. Takashima, S . Katsura. and A. Mizuno, “Lowtemperature NOx Reduction Proccsses Using Combined Systems of Pulsed Corona Discharge and Catalysts”, J. Phys. D:
Appl. Phys. Vol. 34, pp. 604-613, 2001.
[ I l l T. Yamamoto, C. L. Yang, M. R. Beltran, and 2. Kravets,
“Plasma Assisted Chemical Process for NOx Control”, IEEE
Trans. IAS, Val. 36, pp. 923-927, 2000.
1121 M. G. Grothaus, and E. R. Fanick, “Invcstigations of a Pulscd
Corona Reactor for H e d y Duty Diesel Engine Emissions Reduction”, Proceedings 23rd International Powcr Modulation
Symposium, pp. 20-23, Rancho Mirage, CA, 1998.
I131 M. G. Grothaus, M. K. Khair, and P. Paul, “ A Synergistic Approach for the Removal of NOx and PM From Diesel Engine
Exhaust”, 12th IEEE International Pulsed Power Conference,
Digest of Tcchnical Papers, Val. 1, pp. 506-510, 1999.
[I41 B. M. Pencntrate, M. C. Hsio, B. T. Merrit, G. E. Vagtlin, and
P. H. Wallman, “Comparison of Electrical Discharge Techniques for Non-thermal Plasma Processing of NO in N2”, IEEE
Trans. Plasma Science, Vol., 23, pp. 679-687, 1995.
1151 B. S . Rajanikanth, and S. Rout, “Studies a n Nitric Oxide Removal in Simulated Gas Compositions Under Plasma-Dielectric/Catalytic Discharges”, Fuel Processing Technology, Elsevier,
Vol. 74, pp 177-195, 2001.
1161 B. S. Rajanikanth, and V. Ravi, “Removal of NOx from D i e d
Engine Exhaust Using Pulsed Electrical Discharge Coupled with
a Catalytic Converter”, 12th International Symposium on High
Voltage Engineering, India, Vol. 5 , pp. 1283-1286, 2001.
I171 K. Yan, S. Kanazawa, T. Ohkubo, and Y. Namoto, “Oxidation
and Reduction Processes During NOx Removal in N, +O, with
Corona Induced Non-thermal Plasma”, Proceedings, ICESP VII,
KOREA, 1998.