Interpretation of Arterial Blood Gases
advertisement

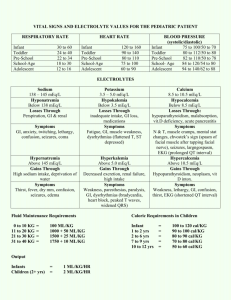
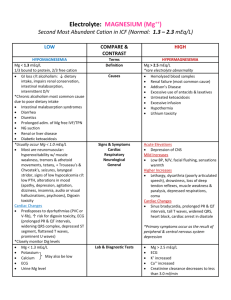
Interpretation of Arterial Blood Gases pH PaCO2 HCO3Base Excess Total CO2 PaO2 Adult Child Newborn Laboratory Normal Ranges 7.35 – 7.45 35 – 45 mm Hg 22 – 26 mEq/L +/- 2 mEq/L 24 – 30 mEq/L Acceptable Therapeutic Ranges 7.30 – 7.50 30 – 50 mm Hg 80 – 100 mm Hg 85 – 100 mm Hg 50 – 70 mm Hg PaCO2 – pH Relationship At a baseline PaCO2 of 40 mm Hg and a pH of 7.40: 1. An acute 10 mm Hg increase in PaCO2 results in a pH decrease of 0.05 unit. For example, if the PaCO2 increases from 40 mm Hg to 50 mm Hg, then the pH will decrease to 7.35 2. An acute 10 mm Hg decrease in PaCO2 results in a pH increase of 0.10 unit. For example, if the PaCO2 decreases from 40 mm Hg to 30 mm Hg, then the pH will increase to 7.50 Determining the Predicted Respiratory pH Determines the expected pH if the only abnormality is the respiratory component. 1. Determine the difference between the measured PaCO2 and 40 mm Hg; then move the decimal point two places to the left. 2. If the PaCO2 is greater than 40, subtract half the difference from 7.40 3. If the PaCO2 is less than 40, add the difference to 7.40 For example: pH 7.04, PaCO2 76 mm Hg (1) (2) (3) 76-40 = 36, move the decimal point 2 places to the left = .36 .36 / 2 = .18 7.40 - .18 = 7.22 pH 7.47, PaCO2 18 mm Hg (1) (2) 40 – 18 = 22, move the decimal point 2 places to the left = .22 7.40 + .22 = 7.62 HCO3- - pH Relationship Under normal circumstances, a 10 mEq/L variance from the normal buffer baseline represents a pH change of approximately 0.15 unit. HCO3BE (Base Excess) pH 24 mEq/L 0 7.40 14 mEq/L -10 7.40 - .15 = 7.25 34 mEq/L +10 7.40 + .15 = 7.55 Determining the Metabolic Component 1. 2. 3. Determine the difference between the measured pH and the predicted respiratory pH. Move the decimal point 2 places to the right. Multiply by two thirds (2/3). Base Excess: Measured pH is greater than the predicted pH Base Deficit: Measured pH is less than the predicted pH For example: pH 7.04, PaCO2 76, predicted pH 7.22 (1) (2) (3) 7.22 – 7.04 = 0.18 0.18 = 18 18 x 2/3 = 12 mEq/L base deficit pH 7.21, PaCO2 90, predicted pH 7.15 (1) (2) (3) 7.21 – 7.15 = 0.06 0.06 = 6 6 x 2/3 = 4 mEq/L base excess Note: On a laboratory report, the base excess or base deficit is usually reported as a positive or negative base excess (BE). For example, a base deficit of 12 mEq/L would be reported as BE = -12 and a base excess of 12 mEq/L would be reported as BE = +12. Guidelines for bicarbonate administration in the adult: 1. 2. 3. 4. Do not routinely treat a base deficit of less than 10 mEq/L. Do not routinely treat an arterial pH greater than 7.20 unless there is cardiovascular instability. When sodium bicarbonate is indicated, calculate as follows: Base Deficit multiplied by weight(kg) / 4 = deficient mEq of bicarbonate Give half the calculated dose and repeat blood gas measurements in 5 minutes. For example: BE = -12 mEq/L, body weight = 70 kg (1) (2) 12 mEq/L x 70 kg / 4 = 210 mEq, Give half the calculated dose: 210 / 2 = 105 mEq