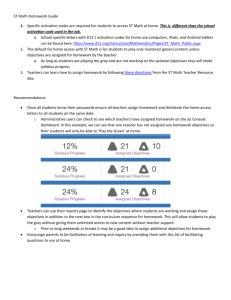
MS Lid%E9n et al Species-specific activation time lags ca n
advertisement

Exploring habitat restrictions in epiphytic lichens 2: Species-specific activation time lags can explain niche differentiation in hydrophilic endangered species. Lidén, M.1, Jonsson Cabrajic, A.2, Ottosson-Löfvenius, M. 1, Palmqvist, K. 2, Lundmark, T. 1 1Unit of Field-based Forest Research/Department of Forest Ecology and Management, Swedish University of Agricultural Sciences, SE-90183 Umeå, Sweden. 2Department of Ecology and Environmental Science, Umeå University, SE-901 87 Umeå, Sweden. Abstract In order to explore underlying physiological causes for habitat restrictions, the main objective of the study was to examine to what extent 1) activation time lags and 2) microclimatic quality in stream habitats may affect realised metabolic activity in endangered hydrophilic epiphytic lichens. To achieve this objective, we characterised species-specific patterns in photosystem activation after hydration and inactivation during desiccation, for four hydrophilic species and one generalist species. Results reveal the occurrence of surprisingly severe activation time lags among the hydrophilic lichens, with full activation being approximated first after 24 h for Usnea longissima and Bryoria bicolor, and after 4 h for Platismatia norvegica and Lobaria amplissima. Subsequently, simulations were performed for all five species using the dynamic model and species-specific model constants presented in Jonsson Cabrajic et al. (ms in prep) on data from 12 stream microhabitats. Simulation results were then used in ANOVAs including the effects of species, site, distance to the stream, and stream quality (rapid vs calm water flow). Results show that for U. longissima, activation time lags can in certain microclimatic conditions reduce photosynthetic output by a factor of five. B. bicolor was almost equally severely affected, while P. norvegica displayed moderate reductions. In contrast, L. amplissima had very good growth prerequisites also in unfavourable microclimates, possibly due to its extreme water-holding capacity. Observed habitat restrictions may therefore be related to sensitivity during e.g. dispersal or juvenile survival. From these results, we conclude that activation time lags may determine whether or not a species may be able to achieve a positive net growth in a certain microhabitat. Recurrently, both close proximity to the streams and presence of rapid water had a strong positive impact on realised activity among the slowly activating species, a pattern that coincides with observed distributional patterns among hydrophilic species. The occurrence of pronounced activation time lags may therefore have provided us with one of the physiological causes behind habitat restrictions in hydrophilic endangered lichens. Äldre version av abstract: In order to explore underlying physiological causes for habitat restrictions, the main objective of the study was to examine to what extent 1) activation time lags and 2) microclimatic quality in stream habitats may affect realised metabolic activity in endangered hydrophilic epiphytic lichens. To achieve this objective, we characterised species-specific patterns in photosystem activation after hydration and inactivation during desiccation, for the hydrophilic species Bryoria bicolor, Lobaria amplissima, Platismatia norvegica, and Usnea longissima, and the generalist species Platismatia glauca. Results reveal the occurrence of surprisingly severe activation time lags among the hydrophilic lichens, with full activation being approximated first after 24 h for U. longissima and B. bicolor, and after 4 h for P. norvegica and L. amplissima. Subsequently, simulations were performed for all five species using the dynamic model and species-specific model constants presented in Jonsson et al. (ms in prep) on data from 12 stream microhabitats. Simulation results were used in an extended factorial habitat analysis, where ANOVAs were performed including the effects of species, site (3 streams), distance to the stream (0,5 vs 20 m) and stream quality (rapid vs calm water flow) as independent variables. Results show that for the most hampered species U. longissima, activation time lags can in certain microclimatic conditions reduce photosynthetic output by a factor of five. B. bicolor was almost equally severely affected, while P. norvegica displayed moderate reductions. Recurrently, both close proximity to the streams and presence of rapid water had a strong positive impact on realised activity among these species. In contrast, L. amplissima had very good growth prerequisites also in unfavourable microclimates, in part due to a very good water-holding capacity. Observed habitat restrictions to humid micro- and macroclimates for this species may therefore be related to sensitivity during e.g. dispersal or juvenile survival. From these results, we conclude that activation time lags may determine whether or not a species may be able to achieve a positive net growth in a certain microhabitat. INTRODUCTION The persistance and growth of lichen populations is in any habitat dependent on the outcome of a rather basic equation. With reference to the physiological traits and limitations of the specific species and population, the biotic and abiotic environment must allow for a successful outcome of all the different phases in the life cycle of the lichen in question. The different parts of this equation are production of reproductive bodies, followed by dispersal and establishment, for which quality specifications often are rather perculiar and success rates very low (e.g. Hilmo 2002; Keon and Muir 2002; Hilmo and Såstad 2001; Sillett et al. 2000; Sillett and McCune 1998; Nilsson and Ericson 1997; Samuelsson et al. 1994; Scheidegger et al. 1995, Hilmo and Såstad 2001). In addition, a positive net growth of the new juvenile thalli has to be achieved. On the level of individual thalli, this first requires that net photosynthesis exceeds net respiration. Second, net growth has to compensate for losses that the thalli suffers in the shape of necrosis (due to parasitic infections and disease resulting from mechanical damage) and fragmentation due to e.g. grazing or damage from wind and snow (Lidén and Hilmo 2002). The long-term persistence of a lichen population in a certain habitat also requires that thalli that manage to reach a reproductive stage will produce enough offspring to compensate for thalli that fail to do so. Thus, positive net growth needs to constitute the core of any analysis of lichen conservation biology. While metabolic activity in lichens is restricted to the periods when the thalli is both hydrated and receiving sufficient irradiation (Hilmo 2002, Palmqvist and Sundberg 2000, Nash 1996, Gaio-Oliveira et al. 2003), studies by Palmqvist and Sundberg (2000) and Dahlman (2003) have also shown that lichen growth is strongly correlated to the irradiation received by presumably active thalli, termed Iwet (mol m-2). Since green-algal lichens in habitats with sufficient air humidity can resume photosynthetic activity after desiccation only by absorption of water vapor (Nash 1996, Lange et al. 1986, 1988; Friedl and Büdel 1996), this factor apparently has a large potential to influence Iwet. High levels of air humidity will also result in a slower desiccation process after rainfall (Gaio-Oliveira et al. 2003). While this implies that lichen growth for many species is likely to be severely hampered in relatively dry continental environments (Palmqvist and Sundberg 2000, Muir et al. 1997, Nash 1996, Halonen et al. 1991, Boucher and Nash 1990), we can also see the potential for elevated levels of microclimatic humidity in e.g. stream habitats to compensate for low precipitation levels of in continental habitats (Lidén and Hilmo 2005). Stream habitats are known to harbour many red-listed hydrophilic epiphyte species (ref). For hydrophilic epiphytic lichens, porophytes adjacent to rapid (turbulent) water might be suitable as substrates for four main reasons. First, higher air humidity levels slow down desiccation after morning dew and rainfall, increasing both total hydration time and the length of single hydration events. Second, aerosols from rapid water may increase length and frequency of hydration events, compared to streams with calm water. Third, around streams, the light climate is generally characterised by higher light availability compared to interior (closed canopy) forest habitats. And, fourth, streams in topographical depressions are likely to feature relatively frequent formation of morning fog, compared to the surrounding terrain. However, while a high canopy openness may increase lichen exposure towards both rain and incident PPFD (both increasing growth potential), the potential positive effect on NP may be counteracted by faster desiccation after hydration events. Although lichen communities often display sharply outlined patterns of niche differentiation among different species, the underlying physiological limitations that restrict species or species groups to certain habitats or substrates are very seldom sufficiently understood, at least not in a mechanistic sense. During desiccation, a series of biochemical reactions result in a shut-down of lichen metabolism. To a different degree in different lichen species, other reactions occur parallel to this process, that serve to protect the photosynthetic apparatus from strains associated with the desiccated state (Brown et al. 1983, Smith and Molesworth 1973, Farrar and Smith 1976). After addition of liquid water, green algal lichens containing Trebouxia photobionts are able to induce 75-80% of maximal photosynthetic electron transport [photosynthesis] and CO2 fixation activity within 10 min, and to reach maximal levels after 30 min (K. Palmqvist, unpublished). In previous studies on lichen growth, it has been assumed that wet and irradiated lichens are fully photosynthetically active (Palmqvist, Dahlman etc xxx). Most studies show that activation in lichens occur rather instantaneously, but a few publications report to have registered up to 1 h with suboptimal metabolic activity subsequent to hydration after liquid hydration (Palmqvist 2000, Lange, Kilian and Ziegler 1986??? mha flytande vatten?). Hereafter, we will refer to this phenomenon as “activation time lags”. One reason behind such delays in activation may be the unfolding of the different physiological protective systems that are formed during desiccation to protect the photosynthetic apparatus of the lichen. This “unfolding process” is not fully understood, but may involve uncoupling of mitochondria (Brown et al. 1983), and repair of membranes (Smith and Molesworth 1973) and/or respirable substrates damaged from membrane desiccation (Farrar and Smith 1976). The elevated levels of respiration connected to this process are referred to as resaturation respiration, resulting in metabolic costs that may be highly variable among species and ecosystems (kolla Lechowicz 1981, Groulx and Lechowicz 1987, Lange 2003a, 2003b as in Lange and Green 2006). Although wetting and drying cycles are essential for the osmotically driven transport of metabolites between mycobiont and photobiont (Kershaw 1985, Nash 1996, Honegger 1998), short hydration events may result in negative NP due to resaturation respiration (Belnap et al. 2004) and leakage of metabolites from the thalli (Dudley and Lechowicz 1987, Honegger 1991). Further, net growth in lichens can be affected by suprasaturation after rainfall, maximal levels of carbon assimilation, and variations in overall respiration levels. Among the factors determining lichen net production, we observe that potential costs related to metabolic activation time lags so far has attracted very little attention. We believe that there are reasons to examine this factor closer, targeting lichen species that are characterised by low NP levels resulting in small, fragile populations. Further, if they are to be found, we hypothesise that species potentially hampered by activation time lags should be overrepresented in the group displaying hydrophilic distributional patterns. In an attempt to assess the influence of high air humidity around streams on potential net growth in lichens with different ecophysiological adaptations, we therefore want to address a specific issue: an slow activation of photosynthesis be a factor that is especially limiting for rare or red-listed hydrophilic lichens? AIM In order to explore underlying physiological causes for habitat restrictions, the main objective of the study was to examine to what extent 1) activation time lags and 2) microclimatic quality in stream habitats may affect realised metabolic activity in endangered hydrophilic epiphytic lichens. To achieve this objective, the present study had three more specific aims. By lab analysis of chlorophyll fluorescence, the first aim was to characterise species-specific patterns in photosystem activation after hydration and inactivation during desiccation, for four red-listed hydrophilic lichen species and one generalist species. The second aim was to use lab data together with field microclimatic characteristics in stream habitats, to perform an extended habitat analysis using the model developed in Jonsson Cabrajic et al. (ms in prep.). The third aim was to use analysis of variance (ANOVA) on the simulation results to quantify and describe the effects of (I) species; (II) distance to the watercourse; and (III) stream characteristics on wet time as well as potential and realised metabolic activity in the studied epiphytic lichens. METHODS STUDY SPECIES AND LICHEN MATERIAL Lab experiments were performed on four red-listed hydrophilic (Bryoria bicolor (Ehrh.) Brodo & D.Hawksw., Lobaria amplissima (Scop.) Forssell., P. norvegica (Lynge) W. L. Culb.& C. F. Culb., and Usnea longissima Ach.) and one common generalist species, P. glauca (L.) W. Culb. & C. Culb. Bryoria bicolor is a fruticose epiphytic lichen growing on xxx trees in xxxx boreal habitats with high air humidity. The species can reach 3-4 cm in length, has xxxx algae, and reproduces xxxx (Brodo et al. 2001). world distribution???, where it is red-listed as xxxx (Gärdenfors 2005). Lobaria amplissima is a foliose epiphytic species with a suboceanic distribution, that typically grows on broadleaf trees in open environments with high air humidity. It has green xxx algae, but also occurs as a cyanobacterial photosymbiodeme ("Dendriscocaulon umhausense") on rock outcrops (Thor and Arvidsson 1999). The species can reach 1 m in diameter, and reproduces rarely by xxxx (Brodo et al. 2001). It is often so scarce on its occurrences that there is a high risk of random extinctions, and is classified as endangered in the Swedish Red List (Gärdenfors 2005). Platismatia norvegica is a foliose, epiphytic species with a suboceanic distribution (Ahlner, 1948, Jørgensen 1996, Holien and Tønsberg 1996) that typically grows on Norway spruce [Picea abies (L.) H. Karst] (Ahlner 1948; Holien 1997) in habitats with either high air humidity or oceanic macroclimates (Lidén and Hilmo 2005, Holien 1997, Hilmo 1994; Holien 1996). It is isidious (Aph. rare), up to 12 (occ. 25) cm, has green trebouxioid alga (Brodo et al. 2001), and is red-listed as vulnerable in Sweden (Thor and Arvidsson 1999). Usnea longissima is a fruticose epiphytic lichen associated with relatively open fire refugial spruce forests with high air humidity. The species can reach 1 (up to 10) m in length, has xxxx algae (Brodo et al. 2001), and reproduces mainly by fragmentation, with rare occurrences of apothecia in relatively humid habitats (??? ref?). U. longissima has declined dramatically the last 50 years, is severely hampered by poor dispersal (Dettki and Esseen xxxx!), and is categorised as vulnerable in the Swedish Red List (Ahlner 1948, Thor and Arvidsson 1999). P. glauca is a thinly lobed foliose lichen which is very closely related to P. norvegica but displays a very different distributional pattern, since it is one of the most common epiphyte in the boreal forests of Sweden, and generally has a very wide habitat tolerance (ref). It has isidia and sorediate isidia, green trebouxioid alga (Brodo et al. 2001), and grows predominantly on Norway spruce while being found also on other tree species. P. norvegica was collected according to Lidén o Hilmo (2005). Lichen material from B. bicolor was collected in the vicinities of (north of Oslo, Norway) in January 2006 (15 jan?). U. longissima was collected from an xx site at xxx, Norway. P. glauca insamlad var? L. amplissima was collected from a fallen tree trunk (33628176 Ö; 6702839 N), at Färnebofjärden in central Sweden after a permit being granted by the county board responsible for the reserve. Immediately after harvest the lichens were air-dried in a desiccator over silica gel at 20.5 C for 24 h, and subsequently stored at -18 C. Prior to measurements, the lichens material was sprayed with water and reactivated for 24 h at 10 C and 2.5 μmol photons m-2s-1 provided by metal halide lamps (??) (HQI-TS 400 W Osram, Berlin, Germany) (ref). Prior to the experiments, thalli were reactivated by placing hydrated lobes on wet Wettex cloths in boxes allowing air circulation under the lid, and pre-conditioned for 24 h at room temperature and low light (2-3 mol m-2s-1) to allow for repair of short-term down-regulation of photosystem II. After 24 h of activation, metabolic activity of all thalli was tested (detailed above). Before the experiments, lichens fulfilling the vitality criteria [beskriv] were again dried over silica gel for 24 h in a desiccator, and subsequently trimmed to a uniform weight for each species, determined to the nearest 0.001 g. Due to natural size differences among the species, L. amplissima, P. glauca and P. norvegica fragments were cut to a dry weight of 0.090 g, U. longissima to 0.050 g, and B. bicolor to 0.020 g, which in the latter case represented fairly large intact thalli. STREAM SITES AND CLIMATE MONITORING Field microclimatic monitoring was performed in 2005 during three measurement campaigns, carried out at three stream sites in the county of Västerbotten in northern Sweden, Kulbäcken, (xxx, yyy, 1122 July), Stenträskbäcken (xxx, yyy, 27 July to 9 August) and Lögdeälven (xxx,yyy, 14 to 24 September). The region belongs to the northern boreal vegetation zone (Ahti et al. 1968) that consists of forests dominated by Norway spruce (Picea abies (L.) H. Karst), Scots pine (Pinus sylvestris L.), and dwarf-shrub or herb-dwarf-shrub communities (sensu Arnborg 1990), and is characterised by a moderately continental climate, with levels of annual precipitation ranging from 450 to xxx mm, and a growing season (mean temperature > 5 C) of 140-160 days/year (Wastensson et al. 1995). The study areas were selected according to the following criteria: 1) presence of a watercourse running through the habitat that presents both calm and rapidly flowing water within 200 m (rapid water microhabitats were not monitored in the present study, but cf. Lidén et al. (ms in prep); 2) that steep topography would not consistently covary with rapid water flow; and 3) that distance from roads would not exceed 0.5 km. Finally, the stream sites were as far as possible (5) selected so that abiotic and habitat-related factors would be as similar as possible among the sites. Since the summer of 2005 was abnormally dry (ref) in the region, the first criteria was hard to fulfil, resulting in the three selected stream sites being situated 60-100 km apart (Fig. x and Table x – basal area, steepness, coordinates). Both Lögdeälven, situated 15 km [?] from the Baltic sea close to Nordmaling (RN xxxx, xxxx) and Kulbäcken, close to Vindeln (RN xxxx, xxxx), are dominated by Downy birch (Betula pubescens Ehrh.) and Grey alder (xxx) with minor occurrences of Norway spruce, while the Stenträskbäcken stream site, situated 50 km north of Lycksele [?] (RN xxxx, xxxx) was dominated by Norway spruce ( 95% of standing volume), with minor occurrences of Downy birch. In order to describe the macroclimatic characteristics of the stream sites, reference normals (1961-1990) for annual precipitation were taken from the meteorological station that was situated closest to the survey site (xxx, Alexandersson et al. 1991; Førland 1993). To assess the importance of stream habitat quality, microclimate was in the present study monitored using a factorial design in a) three different stream environments, including at each stream site the effects of b) proximity to streams (0.5 vs. 20 m), and c) stream flow quality (calm vs. rapid, aerosol-producing water flow), resulting in a total of 12 microhabitats. At each microhabitat, seven fragments of P. norvegica were transplanted at 50 cm distances onto 10 mm mesh size nylon nets. During each measurement campaign, at each of the four microhabitats selected at each stream site, a data logger was placed which in addition to thallus WC also recorded air temperature and relative air humidity. The physical parameters were measured at 1-min intervals and stored as 10-min averages. All microhabitats were visited at least weekly to collect the data, check for broken sensors, and to adjust the WC-clips if necessary. For full details of the experimental setup, general microhabitat characterisation, and routines for quality control of data collection during the monitoring campaign, please see Jonsson Cabrajic et al. (ms. in prep). DETERMINATION OF SPECIES-SPECIFIC ACTIVATION AND INACTIVATION PATTERNS IN RESPONSE TO FLUCTUATING HYDRATION LEVELS After fragment preparation, lab experiments were performed in three different sets for all study species, aiming at quantifying species-specific traits concerning 1) activation of photosynthesis by liquid water, 2) deactivation of photosynthesis during subsequent desiccation, and 3) activation of photosynthesis by humid air. During all laboratory measurements, thallus WC was obtained gravimetrically immediately after each fluorescence measurement occasion. The water potential (ψair) in a given microclimate is the (water) vapour pressure gradient between the thallus and the surrounding atmosphere, which is a direct function of the current relative humidity and temperature. Vpd is the difference between actual vapour pressure in the air, and the value at saturation for the given temperature. In the present study, lab experiments were performed in controlled climatic environments. For clarity, these are below referred to as combination of the specific Vpd and temperature values, although it is the resultant ψair that is the variable later used in simulations with dynamic model presented in Jonsson Cabrajic et al. (ms in prep), and briefly described below in 3.4. General experimental setup Experiments were performed in a flow-through gas-exchange system (Compact Minicuvette System 400, gas mixing unit GMA1 and cuvette GK-022, H. Waltz, Effeltrich, Germany [?]), with temperature regulation controlled by a built-in Peltier element and the illumination of 200 μmol m-2s-1 used during all experiments provided by a xxx Osram lamp.Outside the cuvette, temperature was held at 20.5 C [el ref. Sundberg et al. 1999]. Cuvette experiments were performed at 10C and 15C, in accordance with average temperatures during active time observed by Palmqvist et al. (xxxx) for xxxx, and patterns of preference observed by Green et al. (2002) for xxxx, and by Del-Prado and Sancho (2000) for xxxx. For lichens that in the hydrated state have a relatively high light reflectivity in the thallus surface, the difference ΔTleaf-Tcuv should be comparatively low (Palmqvist and Sundberg 2000). However, to ensure that cuvette temperatures did not deviate significantly from thallus surface temperatures, special test series of ΔTleaf-Tcuv were performed for all species. Since evaporative cooling would prevent temperature increase in hydrated thalli (Lange 2003), comparisons of ΔTleaf-Tcuv were made on air-dry thalli, to obtain maximal levels of deviation possibly affecting the thalli. Typically, differences were in the range of 0,0 – 0,1 grader for the different species and temperature x Vpd combinations, with slightly larger deviations registered for L. amplissima at 10 C x vpd 0,5, and for both L. amplissima and P. glauca at 15 C x Vpds 0,02, 0,1 and 0,5. Different levels of humidity were obtained by transferring 20.5C water vapour saturated air through a cooling trap unit. Photosynthetic activity was in all three sets of experiments indicated by the dark adapted fluorescence yield [alt. maximum capacity of PS2] (Fv/Fm), measured by a PAM-2000 fluorometer (H. Walz, Germany) on dark-adapted thalli (5 min, sufficient duration tested before measurements) (White and Critchley 1999, ev. van Kooten and Snell 1990, review Roháček and Barták 1999). Activation by liquid water and cessation of activity during desiccation In order to monitor activation patterns for the different species, Fv/Fm was followed over time after thallus rehydration (Fig 3), in water vapour saturated air at 10 C and 200 μmol m-2s-1 (level chosen since it corresponds to the light saturation level found for many species at 10C (Palmqvist and Sundberg 2000). All measurements were performed on 4-6 fragments from each species. In the second series, development of Fv/Fm was followed in fully activated thalli fragments during desiccation at eight different combinations of VPD and temperature, in order to monitor the process of desiccation and inactivation in the species. Desiccation was monitored at 10C and 200 μmol m-2s-1 in [ψair corresponding to…] VPDs of 0.02, 0.05, 0.1, 0.2, and 0.5, and at 15C and 200 μmol m-2s-1 in VPDs of 0.02, 0.1, and 0.5. Species specific desiccation time-series in VPD 0.5 for 10 C or 15 C are presented in Fig 2 and 4. and specific parameterised constants presented in Jonsson Cabrajic et al., ms in prep. All measurements were performed on 4-6 fragments from each species. Measurements were repeated at 1 h intervals (as described above) until measured Fv/Fm rates levelled out below a threshold value of 0,1, below which the remaining Fv/Fm pulse was considered to reflect only noise, and photosynthetic activity was considered to have ceased. In all desiccation and liquid activation experiments, fragments were hydrated up to saturation by spraying with liquid water, after which excess water was removed by shaking prior to introduction into the cuvette. Fv/Fm was measured and fragment WC monitored gravimetrically (xxxx precision balance) at fixed intervals, with initial measurement after 10 min followed by 1 h intervals. This interval was adjusted prior to commencing the activation experiments, to ensure that WC in the lichen material did not decline during the process. Additional desiccation series with measurements performed only once, after 6 h, showed that the measurement process contributions to weight loss were negligible (data not shown). To avoid systematic errors due to differences among the species in distribution of activity over the lichen thalli (Barták et al. 2000), and due to expected irregularities in photosystem shutdown (Barták et al. 2005), Fv/Fm was measured by applying six pulses evenly over the thalli, which thereafter were used to calculate an average value. To counteract [loss of excitation] unmeasured areas were covered with a black felt cloth. Activation during water vapour uptake Rehydration kinetics and patterns of activation of photosynthesis by humid air were characterised for all five species by placing desiccated thalli prepared as described above (n = 3 per species) in a 2 L, 13 cm wide and 23 cm high, sealed glass cylinder containing 250 ml of water. The water was continuously stirred on a magnetic stirrer placed in a climate room with a ψair of – 4 MPa (97% RH and 10 °C and low light (2-3 mol m-2s-1). (Fig 1 and 5). Specific parameterised constants are presented in Jonsson Cabrajic et al., ms in prep. During the experiments, the fragments were carefully monitored so that no water condensation occurred on the thalli. During fluorescence measurements, fragments were placed on slightly damp Wettex cloths subjected to the general -4 MPa climate of the room. DATA PROCESSING AND SIMULATIONS After the lab experiments, simulations were performed for all five species using the dynamic model presented in Jonsson Cabrajic et al. (ms in prep). The model used lab results from the present study as a raw material to parameterise species-specific model constants representing water balances and activity patterns for the five species studied here. The constants were utilised in the construction of a dynamic model that, using the ODE solver ode45 in Matlab R2006a, continuously alternates between rehydration and desiccation processes, discriminates between rain and humid air as sources of hydration, and tracks water sources in advance. For full details of parameterisation, model construction and modeling procedure, please see Jonsson Cabrajic et al. (ms in prep). In the present study, the resultant model is used to generate data for use in an extended factorial habitat analysis, where species, distance to the stream and stream quality are included as independent variables. STATISTICAL ANALYSIS Statistical analyses were performed on the habitat simulation results, in combination with parameters characterising the 12 monitored microhabitats on which simulations were performed. In order to determine the effects of habitat, distance from stream, stream quality, and species, ANOVA tests were performed (Minitab General Linear Model) on the dependent variables wet time, potential metabolic activity, and realised metabolic activity. For all dependent variables tests were performed on the total data set, time periods induced by humid air, and time periods induced by rain, resulting in a total of nine dependent variables. When testing these effects, the simulated results for each lichen fragment tested in the lab experiments (see Jonsson Cabrajic et al., ms. in prep) were used as the experimental units, in a model including the random effect of habitat (3 stream sites), the fixed effects of distance from stream (2 levels) and stream quality (2 levels), two-way interactions among the above mentioned variables, and crown cower (%) as a covariate (TABLE 5, different versions). The test was repeated for each species separately, and included a regression analysis of all included effects. Differences were considered significant for p < 0.05. In order to determine whether the significant effects found in the analyses were positive or negative, the Tukey post hoc test was used (p 0.05) (TABLE 5, different versions). Prior to running the tests, the data were tested for homogeneity of variances (Levene's statistic). RESULTS SPECIES-SPECIFIC ACTIVATION RATES IN RELATION TO MICROCLIMATE The lab experiments where lichen fragments were activated at 10C by liquid water, revealed unexpectedly dramatic differences among the studied species (FIG. 3). For P. glauca, photosystem activation (as indicated by Fv/Fm) was near maximum values already after the shortest possible measurement interval of 10 minutes (0,669; 95% of max); 0,677 at 1 h (97%); and 0,671 at 2 h (96%). Also L. amplissima demonstrated a rapid response with Fv/Fm values of 0,535 at 10 minutes (73%); 0,630 at 1 h (86%); and 0,672 at 4 h (91%). In contrast, a remarkably slow activation response was displayed by P. norvegica, with Fv/FM values of 0,244 at 10 minutes (35%); 0,412 at 1 h (60%); and 0,620 at 4 h (90%). Even more extreme metabolic activation time lags were found for the two fruticose hydrophilic species B. bicolor and U. longissima, for which Fv/Fm values stabilised at maximum values only after a full 24 hours. For B. bicolor, Fv/Fm was 0,178 at 10 minutes (32%); 0,256 at 1 h (46%); 0,420 at 4 h (75%); and 0,460 at 15 h (82%). For U. longissima, Fv/Fm was 0,190 at 10 minutes (28%); 0,222 at 1 h (33%); 0,335 at 4 h (50%); and 0,389 at 15 h (58%). Further, the actual maximum values of Fv/Fm differed among the species, being 0,701 for P. glauca (approximated to 90% after 10 min); 0,735 for L. amplissima (appr. after 4 h); 0,691 for P. norvegica (appr. after 4 h); 0,560 for B. bicolor (reached. after 24 h); and 0,675 for U. longissima (reached after 24 h). After activation by humid air for 24 h, relatively low average Fv/Fm values were registered for L. amplissima (0,418) and P. norvegica (0,390), while a gradient of even lower values were obtained by P. glauca (0,303), U. longissima (0,292), and B. bicolor (0,235). When the humid air activation series was finished, it was immediately followed by a complementary 24 h activation period subsequent to addition of liquid water, which resulted in a pattern for this combined approach consistent to the pattern detected in the series including liquid water only as the source of humidity. In this “24 h humid air + 24 h liquid water” activation series, high Fv/Fm values were recorded for L. amplissima (0,689), P. glauca (0,597), P. norvegica (0,710), and U. longissima (0,617), while levels for B. bicolor (0,560) remained comparatively low. SPECIES-SPECIFIC PATTERNS OF HYDRATION AND ACTIVITY DURING DESICCATION Fixa FIGURE 3 EXCEL, different versions: weight (g) vs time (h) (humid air); Fv/Fm vs time (h) (humid air + water): Fv/Fm (% av max) vs WC (%). The desiccation experiments also revealed physiological differences among the species (FIG. 2 and 4). During desiccation at 10C (monitored period 5 h), B. bicolor was the species found to be most sensitive towards dry ψair environments, with cessation of photosystem activity (as indicated by Fv/Fm values below 0,1) after 4 h in VPD 0,1 and 0,2, and after 3 h at VPD 0,5. Also at VPD 0,05 a severe decline in Fv/Fm had occurred after 5 h. (då låg vattenhalten på = ?) For U. longissima and P. norvegica, at VPD 0,5 metabolic activity ceased after 3 h and 2 h, respectively. In contrast, the foliose species P. glauca and L. amplissima remained fully active throughout the monitored period irrespective of VPD environment, with Fv/Fm values ranging from 0,7-0,6 for the different VPD:s, and slightly lowered Fv/Fm values of 0,5 found only for L. amplissima at VPD 0,5. During desiccation at 15C (performed at VPD:s 0,02, 0,1, and 0,5), cessation of photosystem activity was reached even earlier for the same ψair environments. For B. bicolor, activity had in VPD 0,02 declined severely (to Fv/Fm 0,2) already after 3 h, and had in VPD 0,1 and 0,5 ceased after 3 h and 1 h, respectively. For U. longissima and P. norvegica, activity was still maintained at VPD:s 0,02 and 0,1 but ceased earlier at VPD 0,5 - after 1 h for U. longissima and 2 h for P. norvegica. In addition, for P. glauca activity had in the 15C experiment ceased after 3 h at VPD 0,5, while high (0,7-0,6) Fv/Fm values were maintained throughout the monitored period for VPD:s 0,02 and 0,1, which also was the case for L. amplissima in all studied VPD:s. MONITORED AND SIMULATED PATTERNS OF WET TIME FOR P. NORVEGICA IN DIFFERENT MICROCLIMATIC HABITATS In the present study, all mean values for the three streams are based on a total of twelve fragments, three in each of the four microhabitats included per stream (Table 6, cf. Jonsson Cabrajic et al., ms in prep, where one microhabitat per stream is included). When comparing the streams, accumulated wet time for P. norvegica differed rather dramatically as could be expected due to seasonal and weatherrelated differences. Tukey (hsd) tests showed that accumulated wet time during the measurement campaign was significantly higher at Stenträskbäcken (monitored 27 July to 9 August, mean value 10334 min), with lower accumulated values registered at Kulbäcken (11-22 July, 8005 min) and Lögdeälven (14-24 September, 7932 min). For wet time resulting from rain events, all stream sites differed significantly, with the highest accumulated values found at Stenträskbäcken (8107 min), followed by Kulbäcken (4810 min) and Lögdeälven (3183 min). However, the pattern was reversed for wet time resulting from humid air, with the highest levels found at Lögdeälven (4749 min) followed by Kulbäcken (3195 min) and Stenträskbäcken (2227 min), with all three stream sites differing significantly from each other. At Kulbäcken, total wet time for the microhabitats adjacent to calm water was 91,9% of values for the microhabitats adjacent to rapid water. Values for Stenträskbäcken and Lögdeälven were 68,1% and 156%, correspondingly. EFFECTS OF METABOLIC ACTIVATION TIME LAGS ON WET TIME, POTENTIAL AND REALISED PHOTOSYNTHETIC ACTIVITY IN DIFFERENT MICROCLIMATES An overview of the ANOVAs for all studied variables is presented in Table 5. For simulated values for the different species, a full overview for total values, humid air periods and rain periods at each microhabitat is given in Table 6. An overview of the direction (positive or negative) for the significant effects found for different parameters is presented below. Effects of stream site Of the nine studied dependent variables (Table 6), the ANOVA revealed effects of the different stream sites only for total realised activity (Ul and Pn) and total potential activity (Ul). In both cases, the stream site Stenträskbäcken was strongly positive for both species, while the stream site Kulbäcken was negative for Ul. Effects of stream type - rapid vs. calm water flow Positive interaction effects of rapid water and the stream site at Stenträskbäcken were found for total wet time (all species); wet time caused by humid air (La’); wet time caused by rain (all species); total realised activity (La, Pg, Ul); total realised activity caused by rain (all species); total potential activity (all species); and potential activity caused by rain (all species). Positive interaction effects of rapid water and the stream site at Kulbäcken were found for wet time caused by humid air (Bb, Pg, Pn, Ul); realised activity caused by humid air (all species); and potential activity caused by humid air (all species). Positive interaction effects of calm water and the stream site at Kulbäcken were found for wet time caused by rain (La, Pg, Pn, Ul); total realised activity (Pg); total realised activity caused by rain (La, Pg, Pn); and total potential activity (Pg). Positive interaction effects of calm water and the stream site at Stenträskbäcken were found for total realised activity (Bb, Pn). Effects of distance to the stream Close proximity to the stream was found to have a strong positive effect on total wet time (Bb, Pg, Pn, Ul) and wet time (all species) caused by humid air. For the other studied variables, positive interaction effects (* or **) were found close proximity to the stream and either of the sites Stenträskbäcken and Kulbäcken, for wet time caused by humid air (not for La) or rain (all species), and all studied variables related to potential and realised activity (both for all species). Generally, the positive effect of close proximity to the stream was stronger at Stenträskbäcken, where ** dominated with few exceptions. Further, a positive interaction effect between close proximity to the stream and calm water was found for total wet time (Bb, Pg, Pn, Ul); wet time caused by rain (La, Pg, Pn); realised activity caused by humid air (all species); and realised activity caused by rain (Pg). At Kulbäcken, total wet time was at the microhabitats situated 20 m from the stream in average 74,6% of values observed in the microhabitats situated 1m from the stream. At Stenträskbäcken and Lögdeälven, the difference were even more pronounced, with average 20 m values being 66,5% and 61,5% of 1 m values, correspondingly. Effects of canopy cover Negative effects of a high degree of canopy cover were found for wet time caused by humid air (Bb, Pn, Ul); realised activity caused by humid air (all species); and potential activity caused by humid air (Bb, Pg, Pn, Ul). In contrast, positive effects of a high degree of canopy cover were found for wet time caused by rain (all species); realised activity caused by rain; total potential activity (Pg); potential activity caused by humid air (La); and potential activity caused by rain (all species). Effects of species-specific activation time lags on potential vs. realised photosynthetic activity The simulations including the species-specific constants describing metabolic activation time lags are performed only on the daylight parts of the data-sets, i.e. which correspond to the periods between sunrise and sunset. A full overview of values for potential and realised metabolic activity (total, induced by rain, and induced by humid air) during daylight for all five species is given in Table 7, with q values included to describe the quota between potential and realised activity. Also in the extended 12 microhabitat dataset used in the present study (cf. results for three microhabitats in Jonsson Cabrajic et al., ms in prep), differences in q values reveal remarkable differences among the studied species. Irrespective of microhabitat, total values for realised activity for P. glauca and L. amplissima is for rain periods only reduced by 1% (La 5 sites, Pg 6 sites) to 2% (La at one site) compared to potential activity. Also for humid periods, realised activity levels are recurrently very high, being reduced by 1-5% compared to potential activity, with one exception being the 9% reduction of Pg at Kulbäcken Calm 20 m. For P. norvegica, total realised activity for rain periods is only 89% (Kulbäcken Rapid 1m and Calm 1m) to 82% (Lögdeälven Calm 1m) of potential values, and for humid periods 76% (Kulbäcken Rapid 1m) to 62% (Stenträskbäcken Calm 1m), depending on microhabitat. For U. longissima and B. bicolor, metabolic activation time lags reduce realised activity severely compared to potential values. For the two species, q values were for rain periods of 61% (Ul) and 77% (Bb) at Stenträskbäcken Rapid 1m, to 49% (Ul) and 69% (Bb) at Lögdeälven Rapid 20 m, and for humid periods 41% (Ul) and 59% (Bb) at Kulbäcken Rapid 1m, down to the remarkably low levels of 19% (Ul) and 38% (Bb) at Stenträskbäcken Calm 1m. When comparing different microhabitats, the microhabitats at 20 m from the streams have potential activity values for rain periods that are 69% of the values found at 0.5 m from the streams, and realised activity values that are 68% of the 0.5 m values. Also for rain periods, the microhabitats adjacent to calm water have potential activity values that are 83%, and realised activity values that are 82% of rapid water values. Correspondingly, for humid air periods, values at 20 m are 35% (potential) and 34% (realised) of 0.5 m values, and rapid water values are 34% (potential) and 35% (realised) of calm water values. All differences (over all stream sites, for potential and realised activity) among rapid vs. calm water, and 0.5 vs 20 m, were found significant when tested by Tukey (HSD) comparison of means. Generally, differences among the four microhabitats were more pronounced at Stenträskbäcken and Kulbäcken than at Lögdeälven (Table 7). DISCUSSION SPECIES-SPECIFIC PATTERNS OF METABOLIC ACTIVATION TIME LAGS AND WATER-HOLDING CAPACITY In the present study, U. longissima and B. bicolor displayed extremely slow metabolic activation rates, with full activation of photosynthesis being reached first after 24 h for both species. Also for P. norvegica and L. amplissima, rather significant metabolic activation time lags were observed, with full activation of photosynthesis being approximated (to 90%) after 4 h for both species (Figure 3). However, since the curve for P. norvegica is more linear the impact of activation time lag on realised activity will be much larger for this species, as realised activity is proportional to the area below simulated activity curves (Figure 3). Compared to previous findings of activation time lags, with traces of suboptimal metabolic activity registered up to 1 h after liquid hydration (Palmqvist 2000, Lange, Kilian and Ziegler 1986), the four red-listed hydrophilic species in the present study stand out as significantly to severely hampered, with the most severe effects found for U. longissima and B. bicolor. We find no reason to assume that these differences might be due to fragment vitality, since the lichen material from all appeared to be in perfect condition, and had been transported and stored in a similar manner. Thus, our interpretation is that these differences are likely to be due to species-specific photosystem characteristics. Two possible explanations for these observed activation time lags can be that 1) either thylakoid or Calvin cycle enzymes are not sufficiently protected during desiccation, resulting in slow repair processes, and/or 2) that the metabolic systems have been protected sufficiently during desiccation, but the “unfolding” of these protective mechanisms is a very slow process. To complement this picture, results show that U. longissima and B. bicolor are much less capable to hold water within the thalli and to maintain photosynthetic activity at low water potentials, compared to L. amplissima, P. norvegica, and P. glauca (cf. Kappen 1973). The obvious ecological effects of these differences are reflected in the markedly shorter mean wet times obtained in the species-adjusted simulations performed in the present study (Table 6). The ability to hold water obviously aids in prolonging active time for lichens, which may facilitate for the lichen to achieve a positive net growth for the single active period, after initial metabolic costs for resaturation respiration during activation. Thus, one possible interpretation may be that the pronounced activation time lags found especially for U. longissima and B. bicolor protect against unnecessary and costly unfolding of protective systems necessary to cope with desiccation. With the constraints added by metabolic activation time lags, such a strategy would only be functional in habitats where long wet occasions in sufficient light still will be frequent enough to allow for positive net growth. When analysing the distributional patterns of the two species, it is also clear that they are much more abundant in areas with high precipitation rates and an overall oceanic climate, and in areas where microclimatic conditions allow for long periods of hydration during sufficient irradiation (ref websidor artdatabanken + annat). In contrast, for P. glauca and L. amplissima, a low moisture compensation-point and rapid activation of photosynthesis may allow for peaks of positive net photosynthetic gas exchange also during brief hydration events e.g. from dew condensation, as shown e.g. for L. muralis by Lange (2003). Optimal levels of irradiation and WC of course depend on species-specific traits, and trade-offs between maximum levels and total duration of positive NP (Lange 2003, Green et al. 2002) EFFECTS OF MICROCLIMATIC FACTORS ON REALISED PHOTOSYNTHETIC ACTIVITY When comparing total accumulated values for realised activity found in the present study, we can see that values are radically different both among species and among microhabitats (Table 7). For example, at the favourable microhabitat Stenträskbäcken Rapid 1m, values are 42.4 and 41.4, respectively, for the rapidly activating and efficiently water-holding species L. amplissima and P. glauca. For P. norvegica, that has a water-holding capacity equal to that of L. amplissima and P. glauca, but has quite pronounced metabolic activation time lags, the total realised activity value for Stenträskbäcken Rapid 1m is 33.9. Finally, being the two species that are limited both by rapid desiccation and severe activation time lags, total realised activity values for B. bicolor and U. longissima are consequently as low as 27.5 and 20.3 in the same microhabitat. Similar values are found at e.g. Stenträskbäcken Calm 1m. At the least hospitable microhabitats included in the simulations, total realised activity values at Lögdeälven Rapid 20m are 10.0 and 9.5 respectively for L. amplissima and P. glauca, 7.0 for the intermediate P. norvegica, and remarkably low 4.9 and 3.2 respectively for B. bicolor and U. longissima. Similar values are found at Lögdeälven Calm 20m, and slightly higher values repeating the same general pattern are found at Lögdeälven Calm 1m. When separating the effect of activation time lags between activity induced by rain and humid air, we can (as indicated also by the microsites analysed in Jonsson Cabrajic et al., ms in prep) see that realised activity in all species is comparatively more reduced during activity periods induced by humid air. This effect is also most apparent in U. longissima and B. bicolor, the species most severely hampered by metabolic time lags. Palmqvist and Sundberg (2000) found that a higher energy conversion efficiency (1-2%) in studied terricolous species compared to epiphytes (0.4-0.9%) could be attributed to active time, higher mean photon flux densities in the studied microhabitat, and to a certain level of four-fold differences in photosynthetic capacity among the species. In spite of the effect of photosynthetic capacity, the authors emphasise active time as a more important limiting factor than levels of metabolic capacity when active. However, they also point out that "…restriction of metabolic activity to wet occasions imposes an additional limitation, as light fluxes are lower both when it rains, and particularly in the more shaded habitats where many lichens occur". This line of argument further highlights the possible importance of stream habitats, which often provide relatively high levels of both irradiance and air humidity (Brosofske xxxx, + den andra). CAN HABITAT RESTRICTIONS AMONG HYDROPHILIC EPIPHYTIC LICHENS BE EXPLAINED BY ACTIVATION TIME LAGS? Looking at the effects of different habitat features on realised activity in the studied species, a general pattern appears, where microhabitats at 20 m from the streams have potential and realised activity values that are lower than in the microhabitats 0.5 m from the streams. Further, microhabitats adjacent to calm water have both absolute levels of realised activity, and q values that are lower than values obtained adjacent to rapid water. At Lögdeälven, where the rapid water is wide and relatively slow, sun-exposed, and the topography is relatively flat, differences among the four microhabitats are smaller, both for potential activity and realised activity values. The higher mean values for total wet time found at Lögdeälven for the calm microhabitats were solely caused by very high values for wet time caused by dew at the microhabitat at 1 m from calm water, and are thus to be interpreted as an effect of the open habitat at the microhabitat, in which high grass probably also helped prolong the effects of morning dew. Topography can be ruled out as a factor of importance, since incline between the calm water microhabitats only is 0,5 m along the 20 m gradient. Further, a relatively dense canopy cover was positive for realised activity caused by rain, but negative for realised activity caused by humid air. These patterns seem completely reasonable, since a low degree of canopy openness generally will slow down evaporation, while a high degree of canopy openness will facilitate formation of dew and fog during night-time and morning temperature decline. It is well documented that hydrophilic species often are aggregated close to streams (particularly with rushing water), and in dense, wind-shielded habitats. We can observe that distributional patterns in this group coincide very well with the habitat features that generate high realised activity values among the slowly activated species studied here. In the performed simulations, effects of species-specific metabolic activation time lags differ far more among the microhabitats than what was the case in Jonsson Cabrajic et al. (ms in prep), where no effects of stream type or distance to the streams were included into the simulations. While q values for total daylight simulations show that different microhabitats reduce realised activity to a fairly predictive degree among different species, absolute values for both potential and realised activity values differ to a very high degree among the different microhabitats. Observing the extreme differences in realised activity values reported here for different species/habitat combinations (42.4 for L. amplissima at Stenträskbäcken Rapid 1m and 3.2 for U. longissima at Lögdeälven Rapid 20m), we can see that the potential for positive net growth may differ by a factor of 13 times. At Stenträskbäcken Calm 1m, the q of 0.19 value obtained during humid air activation for U. longissima, is straightforwardly translated to mean that for this extreme species, metabolic activation time lags can for certain microclimatic conditions reduce the potential photosynthetic activity by a factor of five. In contrast, L. amplissima displays a highly efficient metabolism, aided both by relatively rapid activation and a very good water-holding capacity. Thus, the species seems to be hampered by some other factor than a poor capacity for positive growth. Observed habitat restrictions to humid micro- and macroclimates for this species must therefore probably be due to inefficient reproduction, dispersal, establishment and/or juvenile survival. This conclusion also feels rather intuitive, given the fact that thalli from this species often become both very thick, and sized as a regular dinner-plate – a characteristic that has resulted in its Latin name. Similar discrepancies between fundamental and realised niches have been found both for L. pulmonaria (Gauslaa et al. 2005) and L. pulmonaria and L. oregana (Antoine and McCune 2004). The fact that species may achieve higher growth rates in high-irradiance environments where they do not occur naturally implies that some other factors than those regulating NP are involved in limiting their distribution. Such factors may be related e.g. to either of the non-adult stages of the life cycle: dispersal, immobilisation (i.e. attachment on the substrate surface), establishment or juvenile survival. Either, the species in question may be microclimatically more sensitive during these stages (with respect to growth potential or sensitivity towards infections and parasitism), or the species may be hampered by poor dispersal during one or several of the same stages. CONCLUSION It is apparent that performances are highly differentiated among the epiphytic lichens characterised in the present study. For three of the four hydrophilic species included, we can see that species-specific metabolic activation time lags, in combination with microclimatic differences - such as the effects of slope gradients as short as 20 m, and/or presence of rapid water - will mould the prerequisites for metabolic performance in a most dramatic manner. Results show that for the most hampered species U. longissima, metabolic activation time lags for certain microclimatic conditions can reduce the potential photosynthetic activity by a factor of five, and that B. bicolor is almost equally severely affected. Also for P. norvegica, habitat features that enhance microclimatic humidity levels may affect metabolic output to a degree that may explain distributional patterns observed e.g. in Lidén and Hilmo (2005). Recurrently, both close proximity to the streams and presence of rapid water had a strong positive impact on realised activity among these species. In contrast, L. amplissima, which is considered to display a hydrophilic distribution pattern, seems to have very good growth prerequisites also in unfavourable microclimates, possibly due to a relatively efficient activation and a very good water-holding capacity. Observed habitat restrictions to humid micro- and macroclimates for this species may therefore be related to sensitivity during e.g. dispersal or juvenile survival. Given the sharp gradients in metabolic efficiency shown in the present study, it seems most reasonable to assume that these may determine whether or not a species may be able to achieve a positive net growth in a certain microhabitat. It is well documented that hydrophilic species often are aggregated close to streams (particularly with rushing water), and in dense, wind-shielded habitats. We can observe that distributional patterns in this group coincide very well with the habitat features that generate high realised activity values among the slowly activated species studied here. The occurrence of pronounced activation time lags, which is reported for the first time in the present study, may therefore have provided us with one of the physiological causes behind habitat restrictions in hydrophilic endangered lichens.