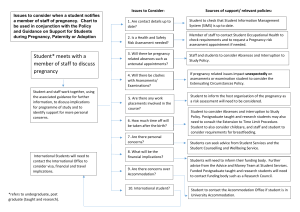
QMUL pregnanacy reporting/ follow-up form
advertisement

JOINT RESEARCH MANAGEMENT OFFICE PREGNANCY REPORTING FORM (BH/QM sponsored trial) Once you have become aware of a Participant or spouse pregnancy, please complete, scan & email/fax this signed form to the GCP Team: 020 7882 7276 (or to the trial co-ordinator’s fax number if multi site project) WITHIN 24 hours of learning of the pregnancy. Please ensure you reviewed and acknowledgment of recipe from the JRMO, print it and file it in your TMF along with the original. Report type: Initial Follow up If the project is multi-site, the section below should be completed by the Main site Trial coordinator prior to sending the template to the sites Full title of the study: Name of sponsor: Sponsor R&D Number: MREC Number: Chief Investigator: BH EudraCT Number: QMUL Is this a double blind study? Name: Email address: Yes Name of ALL IMPs and/or medical devices If Yes are the code break procedures in place with pharmacy? Yes No IMP 1: IMP 3: IMP 2: IMP 4: This section should be completed by the SITE: Subject identification code: Subject or partner : If partner date of consent ( for pregnancy and outcome follow up: DOB: (Day/Month/Year) ( ___ /___/____) Patient’s Age: Principal Investigator: Name: Email address: Trial Co-ordinator local site: Name of reporting host institution: Date of site becoming aware of the event Phone No: No Patient/initials (first, last): _________ Sex: M Name: Email address: Trust/ Institution name: Site number: F Phone No: Phone No: ___/___/____ 1. MATERNAL INFORMATION Date of Birth Date of last menstrual period Expected Date of Delivery Method of contraception: Contraception used as instructed? Yes No Uncertain 2. MEDICAL HISTORY (include information on familial disorders, known risk factors or conditions that may affect the outcome of the pregnancy.) 3. PREVIOUS OBSTETRIC HISTORY (provide details on all previous pregnancies, including termination or stillbirth Pregnancy reporting and outcome form 1.2 Page 1 of 4 Gestation week Outcome including any abnormalities 1. 2. 3. 4. 4. DRUG INFORMATION (list all therapies taken prior to and during pregnancy) Name of Daily Route Date Started Indi Date Treatment drug Dose cati Stopped Start on (week of pregnancy Treatment Stop (week of pregnancy) 5. PRENATAL INFORMATION Have any specific tests, e.g. amniocentesis, ultrasound, maternal serum AFP, been performed during the pregnancy so far Yes No Not known If yes, please specify: Test: Test Date: Result: 6. PREGNANCY OUTCOME Please ensure to collect and report this information to the sponsor within one week of outcome OR within 24 hours if an adverse outcome is learnt (b) Delivery Yes No (a) Miscarriage Yes No If Yes: If Yes Termination of pregnancy Planned Spontaneous Normal Forceps/Ventouse Caesarean If yes please specify elective or emergency Maternal complications or problems related to birth: Please specify the reason and any abnormalities (if known): Date of miscarriage: Date of Delivery: Gestational age at miscarriage: 7. MATERNAL PREGNANCY ASSOCIATED EVENTS If the mother experiences an SAE during the pregnancy, please indicate here and complete an SAE form and submit to JRMO immediately. 8. CHILD OUTCOME Congenital Yes/Nol If any congenital abnormalities, please specify Stillbirth If yes specify date Admission to neonatal intensive care unit If YES reason for admission to the unit Neonatal death Sex: Head circumference: cm Weight¨ kg Yes/No Yes/No Apgar Scores: Height: cm Pregnancy reporting and outcome form 1.2 1 min 5 mins 10 mins Page 2 of 4 9. CHILD OUTCOME FOLLOW UP This form should adapted per study to include child follow-up in applicable trials (see protocol) 10. ASSESSMENT OF SERIOUSNESS (OF PREGNANCY OUTCOME) Life-threatening Mother died Stillbirth/neonate died Date of death Date of death Involved prolonged inpatient hospitalisation Results in persistent or significant disability/incapacity Other seriousness criteria: Congenital Other anomaly/birth significant defect medical Events: 11. ASSESSMENT OF CAUSALITY(OF PREGNANCY OUTCOME) Please indicate the relationship between pregnancy outcome Is the SAE likely to be a IMP X likely or possibly Related Unrelated reaction to one of the IMPs with in the trial? 12. ADDITIONAL INFORMATION: Person completing the form If not the PI specify Name: Phone No: Email address: Signature: Date: Investigator’s Name (PLEASE Print) : Investigator’s Signature Date: For Multi-site trials only Date form RECEIVED by CI’s team from external site: ( ___ /___/____) Date reviewed by sponsor obstetrician ( ___ /___/____) For R&D Office use only Date form RECEIVED by R&D team: ( ___ /___/____) For SUSAR only: CI Reviewed by: Date: Reviewed by: Date: Reviewed by: Date reviewed: Date reported to the MHRA: Pregnancy reporting and outcome form 1.2 Page 3 of 4 Pregnancy reporting and outcome form 1.2 Page 4 of 4