nmr ch3
advertisement

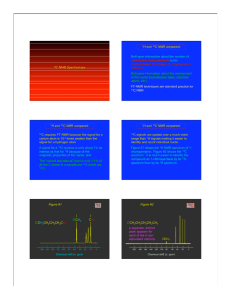
More NMR! 1) Which of the following compounds gives a DEPT-90 spectrum consisting of one signal and a DEPT-135 spectrum consisting of three positive peaks and one negative peak? a) C(CH2CH3)4 b) (CH3)2CHCH(CH3)2 c) CH3CH2CH2CH3 d) (CH3)2CHCH2CH3 2) Propose a structure for a compound which has M+ = 86 in its mass spectrum, an IR absorption at 3400cm-1, and the following 13C NMR spectral data: Broadband decoupled 13C NMR: 30.2, 31.9, 61.8, 114.7, 138.4 DEPT-90: 138.4 DEPT-135: positive at 138.4; negative at 30.2, 31.9, 61.8, 114.7 C5H10O (1 degree of unsaturation) HOCH2CH2CH2CH=CH2 3) Propose structures for compounds with the following formulas that show only one peak in their 1H NMR spectra: a. C5H12 (CH3)4C b. C5H10 cyclopentane c. C4H8O2 cyclohexane with 2 ether O’s across from each other 4) Propose a structure for C7H12O2, which has: Broadband-decoupled 13C NMR: 19.1, 28.0, 70.5, 129.0, 129.8, 165.8 DEPT-90: 28.0, 129.8 DEPT-135: positive at 19.1, 28.0, 129.8; negative at 70.5, 129.0 H2C=CHCOOCH2CH(CH3)2 5) Draw a structure for C8H12O2 1 H NMR: 1.3 (12 H, singlet) Broadband-decoupled 13C NMR: 215.42, 70.37, 18.82 IR: 1750 cm-1 6) Compound A, a hydrocarbon with M+ = 96 in its mass spectrum, has the 13C spectral data that follow. On reaction with BH3 followed by treatment with basic H2O2, A is converted into B, whose 13C spectral data are also given. Propose structures for A and B. Compound A: Broadband-decoupled 13C NMR: 26.8, 28.7, 35.7, 106.9, 149.7 DEPT-90: no peaks DEPT-135: negative peaks at 26.8, 28.7, 35.7, 106.9 (no positive) Vinyl cyclohexane Compound B: Broadband-decoupled 13C NMR: 26.1, 26.9, 29.9, 40.5, 68.2 DEPT-90: 40.5 DEPT-135: positive at 40.5; negative at 26.1, 26.9, 29.9, 68.2 1-cyclohexyl-1-hydroxymethane 7) a) b) c) How many DEPT-90 signals will the above molecule have? 1 How many positive DEPT-135 signals? 2 How many negative DEPT-135 signals? 3