C-13 NMR
advertisement

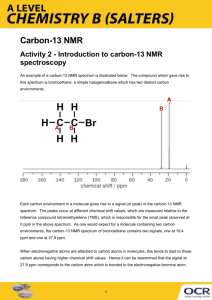
Introduction to C-13 NMR • The 13C nucleus is present in only 1.08% natural abundance. Therefore, acquisition of a spectrum usually takes much longer than in 1H NMR. • The magnetogyric ratio of the 13C nucleus is about 1/4 that of the 1H nucleus. Therefore, the resonance frequency in 13C NMR is much lower than in 1H NMR. (75 MHz for 13C as opposed to 300 MHz for 1H in a 7.04 Tesla field). • At these lower frequencies, the excess population of nuclei in the lower spin state is reduced, which, in turn, reduces the sensitivity of NMR detection. • Unlike 1H NMR, the area of a peak is not proportional to the number of carbons giving rise to the signal. Therefore, integrations are usually not done. • Each unique carbon in a molecule gives rise to a 13C NMR signal. Therefore, if there are fewer signals in the spectrum than carbon atoms in the compound, the molecule must possess symmetry. • When running a spectrum, the protons are usually decoupled from their respective carbons to give a singlet for each carbon atom. This is called a proton-decoupled spectrum. Carbon-13 Chemical Shift Table CC triple bonds http://www.chemistry.ccsu.edu/glagovich/teaching/316/nmr/images/fig15.gif Alkane: 2-methylpentane Alcohol: 2-hexanol OH Alkyl Halide: 3-bromopentane Br Alkene: 1-hexene Aromatic Ring: eugenol O HO Carboxylic Acid: pentanoic acid CO2H Ester: ethyl valerate O O Amide: pentanamide NH2 O Ketone: 3-methyl-2-pentanone O Aldehyde: 2-methylpentanal O H Symmetry in C-13 NMR Each unique carbon in a molecule gives rise to a 13C NMR signal. Therefore, if there are fewer signals in the spectrum than carbon atoms in the compound, the molecule must possess symmetry. Examples: CH3CH2 CH2CH3 OH Enantiotopic vs Diastereotopic CH3’s O enantiotopic OH CH3 O diastereotopic * * * ** Determine the number of signals in the proton-decoupled C-13 NMR spectrum of each of the following compounds: O O H3C CH3 CH3 OCCH3 HO OCH3 N H CH3 OH OH CH3 H3C CH3 Carbon-13 NMR Spectrum of Geraniol ppm 139.07 131.62 124.07 123.71 59.16 39.64 26.51 25.66 17.66 16.24 Carbon # 1 2 3 4 5 6 7 8 9 10 8 9 T1 and NOE Effects in C-13 NMR Because of unequal T1 and NOE effects, peaks heights vary widely in C-13 NMR. This is why C-13 spectra are normally not integrated. CH3 1 3 2 2 3 4 Carbon T1 (sec) NOE CH3 16 0.61 1 89 0.56 2 24 1.6 3 24 1.7 4 17 1.6 4 1 CH3 Carbon-13 Proton-Coupled Patterns http://www.chemistry.ccsu.edu/glagovich/teaching/316/nmr/13ccoupled.html Carbon-13 Proton-Coupled Spectrum of Ethyl Phenylacetate Difficult to interpret O O C=O Typical coupling constants for 13C-1H onebond couplings are between 100 to 250 Hz. http://www.chemistry.ccsu.edu/glagovich/teaching/316/nmr/13ccoupled.html DEPT Spectra DEPT-135 DEPT-90 DEPT-45 CH C CH2 CH3 normal C-13 spectrum Quaternary carbons (C) do not show up in DEPT. Simulated DEPT Spectra of Ethyl Phenylacetate O O DEPT-135 O DEPT-90 O DEPT-45 Normal C-13 spectrum DEPT Spectra of Codeine Predict the normal C-13, DEPT-90, and DEPT-135 spectra of ipsenol, whose structure appears below. DEPT Spectra of Ipsenol DEPT-90 DEPT-135 CDCl3 Normal C-13 spectrum www.lasalle.edu/~price/DEPT%20and%20COSY%20Spectra.ppt Determine the number and appearance of the signals in the DEPT-45, DEPT 90, and DEPT 135 NMR spectrum of each of the following compounds: O O H3C CH3 CH3 OCCH3 HO OCH3 N H CH3 OH OH CH3 H3C CH3