Unit 3 - Bioenergetics Objectives and Essay Samples
advertisement

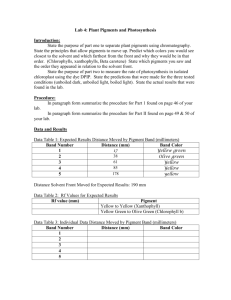
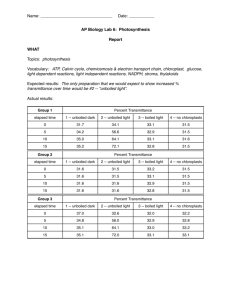
AP Bio – Unit 3 Bioenergetics Objectives Chapter 8 NAME HOUR Do p. 162 #1-7 Section 8.1(p 142-145) – do CC 8.1 p. 145 1. Define biochemical pathway, and give examples. Explain how some biochemical pathways are regulated. 2. Explain the concepts and the consequences of the first law and the second law of thermodynamics. Section 8.2 ( p146-149) – do CC 8.2 p.149 3. Evaluate graphs of reaction progress (free energy changes): o Distinguish between endergonic and exergonic reactions. o Determine the amount of energy stored or released by a reaction. o Determine the activation energy of a reaction with and without a catalyst. o Predict approximate rate of reaction. Section 8.3 (p149-151) – do CC 8.3 p. 151 4. Explain how energy is stored, released, and transferred by ATP. Sections 8,4 & 8.5 (p152-161) – do CC 8.4 p.157 and CC 8.5 p.160 5. Explain how enzymes perform their function. o Describe the role of coenzymes, cofactors, activators and inhibitors in regulating enzyme activity. o Identify optimal conditions for enzyme activity. o Explain how differing conditions might change the ability of an enzyme to function. o Identify conditions that might denature an enzyme. Chapter 9 Do p. 183 #1-7 Section 9.1 (p163-168) – do CC 9.1 p.168 6. Distinguish between oxidation and reduction reactions. Give biologically significant examples. 7. Recognize the several types of individual reactions which may take place in a biochemical pathway 8. Summarize the overall cellular respiration reaction and explain its significance to living cells 9. Summarize each component reaction of cellular respiration and explain how each contributes to the larger process Section 9.2 (p 168-169) – do CC 9.2 p. 169 10. Explain the process of glycolysis in detail Section 9.3 (p170-172) – do CC 9.3 p. 172 11. Explain how pyruvate is oxidized in eukaryotic organisms under aerobic conditions Section 9.4 (p172-177) – do CC 9.4 p. 177 12. Explain the process of oxidative metabolism ("Kreb's Cycle") in detail 13. Explain how chemiosmosis is used to release energy from hydrogen, and store the energy by forming ATP Section 9.5, 9.6 (p177-181) – do CC 9.5 p. 181 14. Define fermentation. Describe several common types of fermentation (ethanol, lactic acid, acetic acid) Note: type of organism; availability of oxygen (aerobic/anaerobic) 15. Explain how cell metabolize foods other than carbohydrates Chapter 10 Do p.205 #1-9 Section 10.1(p184-189) – do CC 10.1 p. 189 16. Outline the overall process of photosynthesis and explain how each metabolic pathway contributes to the overall reaction 17. Define pigment. List several plant pigments and explain their role in photosynthesis Section 10.2 (p189-197) – do CC 10.2 p. 197 18. Explain the energy fixing reactions in detail. Distinguish between cyclic and noncyclic photophosphorylation Section 10.3 p198-199) – do CC 10.3 p. 199 19. Explain the carbon fixing reactions (Calvin Cycle) in detail Section 10.4(p199-203) – do CC 10.4 p. 202 20. Define photorespiration. Explain why it occurs and what conditions make it more likely to occur 21. Explain some adaptations that plants have to overcome the problem of photorespiration Note: Leaf anatomy; C4 photosynthesis; CAM photosynthesis 22. Relate each anatomical adaptation to its corresponding biochemical adaptation (how does its structure match its chemistry) and explain the type of environment in which each would gain the most advantage AP BIOLOGY – CELLULAR ENERGETICS ESSAY SAMPLES Cellular Respiration Essays 1977 Explain how the molecular reactions of cellular respiration transform the chemical bond energy of Krebs cycle substrates into the more readily available bond energy of ATP. Include in your discussion the structure of the mitochondrion and show how it is important to the reactions of the Krebs cycle and the electron transport chain. 1995 Energy transfer occurs in all cellular activities. For 3 of the following 5 processes involving energy transfer, explain how each functions in the cell and give an example. Explain how ATP is involved in each example you choose. Cellular movement Active Transport Synthesis of Molecules Chemiosmosis Fermentation Photosynthesis Essays 1978 Explain how the molecular reactions of photosynthesis transform light energy into chemical bond energy. Include in your discussion the relationship between chloroplast structure and light and dark reactions. 1979 In relation to plants, describe in detail one way of: a. measuring the rate of transpiration b. measuring the rate of photosynthesis c. separating pigments 1986 Describe the light reactions of photosynthesis and, for both a C3 and C4 plant, trace the path of a carbon dioxide molecule from the point at which it enters a plant to its incorporation into a glucose molecule. Include leaf anatomy and biochemical pathways in your discussion of each type of plant. 2004 A controlled experiment was conducted to analyze the effects of darkness and boiling on the photosynthetic rate of incubated chloroplast suspensions. The dye reduction technique was used. Each chloroplast suspension was mixed with DPIP, an electron acceptor that changes from blue to clear when it is reduced. Each sample was placed individually in a spectrophotometer and the percent transmittance was recorded. The three samples used were prepared as follows. Sample 1 – chloroplast suspension + DPIP Sample 2 – chloroplast suspension + DPIP surrounded by foil wrap to provide a dark environment Sample 3 – chloroplast suspension that has been boiled + DPIP Percent Transmittance in Three Samples Light, Unboiled Time (min) % Transmittance Sample 1 Dark, Unboiled % Transmittance Sample 2 Light, Boiled % Transmittance Sample 3 0 28.8 29.2 28.8 5 48.7 30.1 29.2 10 57.8 31.2 29.4 15 62.5 32.4 28.7 20 66.7 31.8 28.5 a. On the axes provided, construct and label a graph showing the results for the three samples. b. Identify and explain the control or controls for the experiment c. The differences in the curves of the graphed data indicate that there were differences in the number of electrons produced in the three samples during the experiment. Discuss how electrons are generated in photosynthesis and why the three samples gave different transmittance results 2005 Yeast cells are placed in an apparatus with a solution of sugar (a major nutrient for yeast metabolism). The apparatus detects bubbles of gas released by the yeast cells. The rate of respiration varies with the surrounding temperatures as indicated by the data below: Temperature (degrees Celsius) 0 10 20 30 40 50 60 70 Number of bubbles of gas produced per minute 0 3 7 12 7 4 1 0 a. Graph the results on the axes provided. Determine the optimum temperature for respiration in the yeast. b. Respiration is a series of enzyme-catalyzed reactions. Using your knowledge of enzymes and the data above, analyze and explain the results of this experiment. c. Design an experiment to test the effect of varying the pH of the sugar solution on the rate of respiration. Include a prediction of the expected results