Scope statement
advertisement

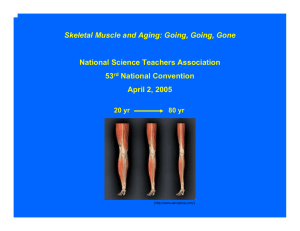
Sarcopenia Guideline Development Scope Statement A ‘Clinical Guideline’ includes recommendations on the appropriate treatment and care of people with specific diseases and conditions. They are based on the best available evidence. The scope statement presented here defines what the guideline “Sarcopenia - prevention and treatment” will (and will not) examine, and what the guideline developers will consider. It also defines the areas of care for which specific statements and measures will be developed. 1. Justification 1.1. Epidemiology 1. Sarcopenia has been suggested in 1988 by Rosenberg to describe an age-related decline in muscle mass [1]. Such atrophy of the skeletal muscle starts around the age of 30 [2] (mostly without symptoms), increases with age and may aggravate in clinical situations (disease, malnutrition, immobilisation,…). Primary sarcopenia is defined as ‘age-related’ without other causes, while secondary sarcopenia may present earlier in life when one or more causes (e.g. sedentary lifestyle, acute inflammatory disease and inadequate dietary intake) are evident [3]. An important consequence of muscle atrophy is the loss of muscle strength. Interestingly, the age-related loss of muscle strength cannot be explained by muscle atrophy alone and thus other factors contribute to sarcopenia [4]. To describe the age-related loss of muscle strength independently from sarcopenia, Manini et al. suggested the term “dynapenia” [5, 6].” The European Working Group on Sarcopenia in Older People (EWGSOP) takes into consideration the presence of low muscle mass and low muscle function (strength or physical performance) to establish whether or not a patient suffers from sarcopenia [3]. 2. At some point in life, both the loss of muscle mass and strength may cross a ‘clinical’ boundary, leading to physical disability [4, 6]. People aged 70 and older live near their maximal capacities and simple tasks (e.g. chair rising) require efforts above 80% of the maximum capabilities [7]. Consequently, these people are prone to physical disability due to supplementary muscle weakness that may be induced by pathology. 3. The consequences of sarcopenia in older people are serious and life-changing: it has an impact on morbidity, disability, health care costs and mortality [3]. 4. A range of lifestyle, pharmacological, non-pharmacological and rehabilitation interventions are reported to prevent and/or treat sarcopenia. 1.2. Current practice 1. Initially, sarcopenia is presented without symptoms and therefore people may not be aware of it. In later stages sarcopenia has been shown to be associated with disability. 2. At the moment, no well-defined care pathway for sarcopenia exists. 1 3. Research in gerontology and geriatrics exploded the last decade, thus leading to fundamentally new insights and knowledge regarding ageing processes, strategies to improve successful ageing and good geriatric practice. In order to implement new strategies in daily practice, there is a need for an appropriate translation of recent scientific findings into realistic and feasible recommendations. 2. Objectives / Deliverables The aim of the Sarcopenia Guideline Development project is to translate the actual scientific body of knowledge regarding Sarcopenia into a practice guideline which fits the Belgian context (e.g. availability of different services for older people or reimbursement of screening tests). Therefore recommendations will be written in French and Dutch and will focus on three levels: 1) Recommendations for health care and prevention specialists; 2) Summary of Evidence; 3) ‘Information for the public’ (Layman’s terms). 3. Guideline 3.1. Population 1.1.1. Groups that will be covered a) Healthy older people who remain above the cut-off values of the EWGSOP diagnostic criteria b) Older people with muscle mass below the cut-off values of the EWGSOP diagnostic criteria but without impact on muscle strength or physical performance (EWGSOP presarcopenia) c) Older people with low muscle mass, plus low muscle strength and/or low physical performance (EWGSOP sarcopenia) 1.1.2. Groups that will not be covered a) People aged under 65 years 3.2. Settings All relevant settings that are involved in the promotion of active and healthy ageing and care for older people. 3.3. Management 1.1.3. Key issues that will be covered a) Risk factors b) Screening & assessment including: a. Muscle function (Muscle strength, Grip strength) b. Muscle mass (Bio impedance,…) c. Functional performance (Standing Balance tests, Sit-to-stand time, Gait speed,…) c) Pharmacological treatment including: 2 a. Vitamin D b. Testosterone and other hormones (e.g. growth hormone) c. ACE inhibitors d. Anti-inflammatory and/or immunomodulatory drugs e. Myostatin or ActIIreceptor antibodies f. Not proven anti-ageing therapies (e.g. creatine) d) Non-pharmacological treatment including: a. Exercise and physical activity (including barriers & motivators to initiate, adhere and change related lifestyle) b. Caloric & protein supplementation (including barriers & motivators to initiate, adhere and change related lifestyle) 1.1.4. Key issues that will not be covered Influence of genetic or other non modifiable factors Metabolic muscle function (non insulin mediated glucose uptake) a) b) c) d) e) f) g) 1.1.5. Main outcomes Muscle performance (mass, strength, endurance, flexibility) Morbidity Disability Mortality Quality of life Function and participation Adverse events 1.1.6. Economic aspects Developers will take into account both clinical and cost effectiveness when making recommendations involving a choice between alternative interventions. 4. Areas of care that will be considered a) Prevention b) Treatment 5. Product acceptance criteria A consensus-based guideline development procedure, empowered by scientific evidence, will be used. Level of evidence will be assessed and indicated for all recommendations. 3 Bibliography 1. 2. 3. 4. 5. 6. 7. Rosenberg, I.H., Sarcopenia: origins and clinical relevance. J Nutr, 1997. 127(5 Suppl): p. 990s991s. Lexell, J., C.C. Taylor, and M. Sjostrom, What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83year-old men. J Neurol Sci, 1988. 84(2-3): p. 275-94. Cruz-Jentoft, A.J., et al., Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing, 2010. 39(4): p. 412-23. Bautmans, I., K. Van Puyvelde, and T. Mets, Sarcopenia and functional decline: pathophysiology, prevention and therapy. Acta Clin Belg, 2009. 64(4): p. 303-16. Clark, B.C. and T.M. Manini, Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci, 2008. 63(8): p. 829-34. Manini, T.M. and B.C. Clark, Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci, 2012. 67(1): p. 28-40. Hortobagyi, T., et al., Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci, 2003. 58(5): p. M453-60. 4