USC Infectious Waste Management Plan
advertisement

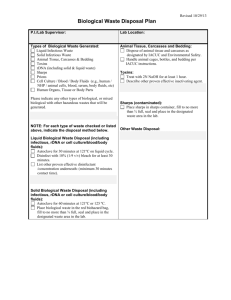
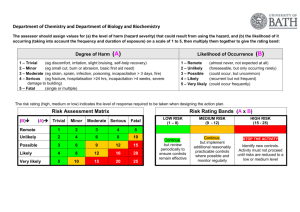
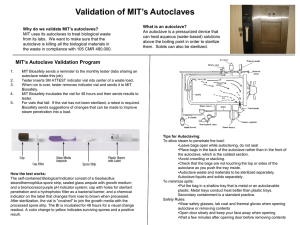
Infectious Waste Management Plan USC Health & Safety Programs Unit 777-2839 POLICY: A. In keeping with the University of South Carolina's policy of providing protection for its employees, students, visitors and community, a program has been developed for USC that describes the procedures for the identification, treatment, packaging, storage, transportation, and disposal of infectious wastes generated within the confines of USC. This policy aims to minimize risk to staff, public, and the environment from improperly handled infectious waste and to plan for any infectious waste emergencies. B. General Information 1. Infectious waste is defined as any waste (solid or liquid) that is capable of producing an infection. These wastes are characterized by the known or suspected presence of pathogens. 2. All persons required to handle infectious waste or materials will be provide with appropriate orientations, personal protective equipment, Hepatitis B vaccination, and onthe -job training. 3. Each department that generates or handles infectious waste will write specific policies and procedures that contain information regarding the identification, safe handling, treatment, packaging, storage, transportation, and disposal of these wastes. The policies and procedures for these departments will be reviewed and approved annually by the infectious control committee. PROCEDURE: A. Designation of Infectious Waste 1. Infectious waste will be classified as infectious by the Infection Control Committee. At a minimum the following will be classified as infectious: a. Sharps - all syringes and hypodermic needles used or unused. All contaminated broken glassware, microscopy slides, pipette tips, pasteur pipettes, etc. b. Microbiologicals - all cultures and stocks of infectious agents; discarded live and attenuated vaccines; cultures dishes/devices used to transfer, inoculate, and mix cultures. c. Blood and Blood Products - all waste unabsorbed human blood, or blood products, or absorbed blood when the absorbent is supersaturated, including but not limited to: serum, EHS-M-013 Page 1 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ plasma and other components of blood, and visibly bloody body fluids such as suction fluids, excretions, and secretions. d. Pathological Waste - All tissues, organs, limbs, and other body parts removed from the whole body, and body fluids to which Universal Precautions apply (cerebrospinal fluid, synovial fluid, pleural fluid, peritoneal fluid, pericardial fluid, amniotic fluid, semen, and vaginal secretions. e. Contaminated Animal Waste - Animal carcasses, body parts and bedding when the animal has been intentionally exposed to human pathogens in research or the production of biologicals. f. Other Waste - Any other material designated by written generator policy as infectious or any other materials designated by a generator as infectious by placing the waste into a container labeled infectious. Any solid waste, which is mixed with infectious waste, becomes designated as infectious waste and must be so managed. 2. The classification of infectious waste will be reviewed and revised annually by the Infection Control Committee. B. Segregation and Packaging 1. Infectious waste will be segregated from other waste at the point of origin by placing it in containers that are impervious to moisture. The containers will not be allowed to become so full that the top cannot be closed. 2. All sharps will be placed in rigid, puncture-resistant and leak resistant containers. 3. Infectious waste will be contained in clearly marked bags or other containers that are red or orange in color. Autoclave bags may be placed directly in the accumulation boxes provided. 4. The biological hazard symbol will be used to mark these containers. All containers must be labeled with the words: "infectious waste, "biohazardous waste" or " medical waste" written in English. 5. All waste packages must have a completed tag filled out. and attached. These tags will be available though your department's Health and Safety Coordinator, or the Health and Safety Department. C. Treatment 1. A written quality assurance plan must be implemented when conducting any on site treatment. EHS-M-013 Page 2 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ 2. Stream Sterilization (Autoclaving) - The following waste will be sterilized before disposal: * Culture plates & Stocks * Any infectious waste that must be stored longer than 92 hours at room temperature. DHEC has outline rules and procedures when using steam sterilization as a treatment method. This procedure is in Appendix A. 3. Compactors or grinders will not be used to process infectious waste. 4. All biohazardous waste (treated or untreated) will be picked up be the contractor to be incinerated. No biohazardous waste will be land filled. D. Accumulation Point 1. All sites that generate large amounts of biohazardous waste will be assigned an accumulation point. This area must be protected from animals, weather and public. The area must be labeled and access limited. 2. All biohazardous waste brought to the accumulation point must be properly package as described above and be tagged. 3. The waste can then be placed in the accumulation boxes and logged in. 4. When a box is nearing capacity, please notify the Health and Safety Coordinator. E. Off Site Disposal 1. All infectious waste treated or untreated will be collected, transported , and stored in the manner previously described agreed upon by our facility and the licensed transporter. 2. The contractor will pick up and transport the infectious waste in leak-proof, fully enclosed containers to a site approved by all regulatory bodies for handling and disposing of infectious waste. 3. All containers to be shipped out must be labeled "USC - (NAME OF CAMPUS) and DHEC #. 4. The Hazard Materials Coordinator will be sure all manifest for these waste transported off site are completed when necessary and kept on file. 5. It is the responsibility of the contractor to maintain all valid permits relevant to disposal of infectious waste. EHS-M-013 Page 3 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ F. Contingency Planning 1. All spills of infectious waste will be cleaned up immediately by a properly protected person trained in the appropriate procedures. 2. All spill residue, including broken glass, will be disposed of as infectious waste. Broken glass should be removed carefully. 3. During clean - up all personnel will wear proper personal protective clothing and equipment, including goggles, lab coat, face mask, and gloves. 4. A spill should be identified with a warning sign so that others in the area will not be contaminated. 5. Liquid spills of less than 5 ml or 5 gm should be covered with absorbent gauze pads and covered with liquid disinfectant. Solids should be wiped with wet absorbent gauze. The spill areas than should be cleaned using a disinfectant solution followed by clean water. 6. For spills of more than 5 ml or 5 gm, spread should be limited by gently covering with absorbent sheets or spill control pads and covered with liquid disinfectant. Access to the area should be restricted. 7. All contaminated surfaces should be thoroughly cleaned with a detergent solution and then wiped with clean water. All contaminated adsorbents and other materials should be disposed of as infectious waste. EHS-M-013 Page 4 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ Appendix A Stream Sterilization Protocol All sharps must be place in a rigid, puncture - resistant and leak - resistant container. All other biohazardous waste must be placed in a red or orange bag clearly labeled with the biohazard symbol. Stream Sterilization is considered an approved method to treat certain types of biological waste. All biological cultures must be stream sterilized before disposal. After treatment by stream sterilization the waste is no longer biohazardous and can be treated as regular waste with the exception of sharps. All sharps should be incinerated. To use stream sterilization the following must be adhered to: 1. Record the temperature and time during each complete cycle to ensure the attainment of a temperature of 121ø C for 45 minutes of longer at fifteen pounds pressure, depending on quantity and density of the load in order to achieve sterilization of the entire load. 2. The steam sterilizer must have a gauge, which indicates the pressure of each cycle. 3. Use heat sensitive tape or other device for each container that is processed to indicate that the steam sterilization temperature has been reached. 4. The stream sterilizer must be adequately vented. EHS-M-013 Page 5 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ Appendix B USC Autoclave Safety Policy An autoclave is a commonly used piece of equipment in biomedical laboratories. Autoclaves pose many hazards including physical hazards (e.g. heat, steam and pressure) and biological hazards. This policy is intended to provide practical information that can be utilized by all researchers to safely operate the autoclaves at USC. Individual labs are encouraged to use this policy as a guide for training new personnel on the safe use of autoclaves. Controls for different brands of autoclaves may have unique characteristics for loading, load sizes, and cycle types and settings. The type of materials you sterilize will determine the type of sterilization cycle you use. For this reason, it is important to review and understand the owner’s manual before using any autoclave for the first time. Always ensure the owner’s manual is readily available in case questions or concerns arise during operation. General Autoclave Safety Practices: 1. Before using the autoclave, check inside the autoclave for any items left by the previous user that could pose a hazard (e.g. sharps). 2. Clean the drain strainer before loading the autoclave. 3. Load the autoclave properly as per the manufacturer’s recommendations. 4. Individual glassware pieces should be within a heat resistant plastic tray on a shelf or rack and never placed directly on the autoclave bottom or floor. 5. Make sure the door of the autoclave is fully closed (latched) and the correct cycle has been selected before starting the cycle. 6. When the cycle is complete, open the door slowly. Keep your head, face, and hands away from the opening. 7. Wear heat-resistant gloves when opening the autoclave door after a cycle. If there is a sharps hazard (e.g. biological waste), wear heat AND cut resistant gloves. 8. At a minimum, when removing items from an autoclave, a rubber apron, rubber sleeve protectors and heat-resistant gloves should be worn. 9. Do not autoclave items containing corrosives, solvents or volatiles or radioactive materials. Additional Practices for Autoclaving Liquids: 1. When running an autoclave cycle with liquids, the cycle time is longer, but uses lower temperatures to minimize evaporation of the liquids being sterilized. EHS-M-013 Page 6 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ 2. To prevent bottles from shattering during pressurization, the caps of containers with liquids must be loosened before loading. 3. Use only borosilicate glass (PyrexTM or KimaxTM) which can withstand the high autoclave temperature. 4. Use a tray with a solid bottom and walls to contain the contents and catch spills. 5. Before removing autoclaved items, wait 10 minutes for autoclaved liquid loads. 6. Let liquids stand for a full hour before touching with ungloved hands. Be sure others in the area know a heat hazard is present. Additional Practices for Autoclaving Dry Loads: 1. Add 1/4 to 1/2 inch of water to the tray so the bottles will heat evenly. 2. Check plastic materials to ensure they are compatible with the autoclave. 3. Before removing autoclaved items, wait 5 minutes for loads containing only dry glassware. 4. For dry loads, let the glassware cool for 15 minutes before touching it with ungloved hands. Autoclave Monitoring & Maintenance: Autoclave monitoring and maintenance is an important aspect of a properly functioning autoclave. Follow the manufacturer’s recommendations for preventative maintenance and ensure all contractors are approved by the manufacturer. Autoclave operators should ensure that each autoclave is monitored as follows: Heat Sensitive Tape Monitoring – Operators should use heat-sensitive sterilization indicator tape for each load to indicate the load has undergone an effective steam sterilization process. This tape only indicates that the proper temperature has been reached, but does not indicate it was heated for the proper length of time. Additional monitoring is not required because all red bag infectious waste is picked up by EHS after autoclaving, and removed from campus by a contracted firm for incineration. Recordkeeping: Operators should maintain documentation of any autoclave preventative maintenance or repairs. These records should indicate who performed the work, the type of maintenance or repairs conducted, and the date the autoclave was serviced. The records should be maintained either in the room with the autoclave, or signage should be posted indicating the location of any records that document autoclave maintenance or repairs. EHS-M-013 Page 7 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_ Training: Each laboratory must develop and implement an autoclave safety training program. All users must be trained before operating an autoclave and the laboratory PI/supervisor is responsible for ensuring each person in the lab is appropriately trained. All training must be documented and records should be maintained in the lab with your other safety training certificates. The laboratory PI/supervisor is encouraged to use this policy as a guide for training new personnel. Autoclave Failure: Discontinue use immediately if an autoclave is not working properly. Post a sign alerting others not to use the autoclave. Mechanical failure needs to be attended by a trained technician. Contact the service company responsible for maintenance of your autoclave or your department’s safety representative for further guidance. Burn Emergency: If you are burned, you should seek medical treatment as soon as possible. Burns to the face, third-degree burns, or burns over large areas of the body should be treated as emergencies. Minor burns should be treated by using first aid procedures. These procedures would include immersing the burn in cool water immediately, removing clothing from the burn area, and keeping the injured area cool for at least five minutes (preferably longer). Any burns to the face or eye or any burns that blister should be seen by a physician. Regardless of the degree of severity, report the burn to your lab supervisor or Principal Investigator as an occupational injury. EHS-M-013 Page 8 Destroy Previous Revisions Issued Date: 2/14/11 Approved: _RWW_