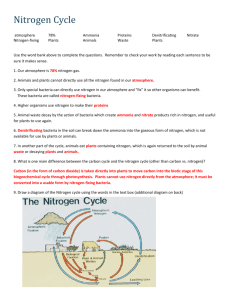
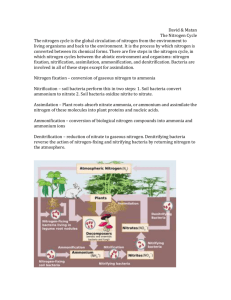
Nitrogen is present in the protein and nucleic acids in animals
advertisement

Nitrogen is present in the protein and nucleic acids in plants. Nitric acid is formed when oxides of nitrogen react with water in the atmosphere. The Nitrogen Cycle Chemosynthetic organisms obtain the energy for fixing carbon dioxide from the conversion of nitrite to nitrate. Nitrogenous fertilizers are made from ammonia by an industrial process. Burning fuels produces polluting oxides of nitrogen. The enzyme nitrogenase converts nitrogen into ammonia. The ammonia is then converted into protein. denitrification nitrogen fixation nitrification industrial fixation Nitrogen in the atmosphere Nitrites (NO2-) Nitrates (NO3-) Ammonium (NH4+) lightning Free-living blue-green algae and bacteria in the soil. Symbiotic blue-green algae and bacteria in nodules of legumes. Nitrate is taken in by root hair cells and transported to growing points. Lightning acts on oxygen and nitrogen producing oxides of nitrogen. Nitrogen and hydrogen are reacted together in the Haber process, producing ammonia. Proteins are decomposed via amino acids to ammonia. runoff defaecation excretion Denitrifying bacteria use nitrate as an electron acceptor in anaerobic conditions. Rain washes soluble nitrates out of soils into rivers and oceans. Nitrogenous fertilisers are applied to fields to enhance crop growth. absorption assimilation decay ammonification denitrifying bacteria death Nitrogen is present in the protein and nucleic acids in animals. Chemosynthetic organisms obtain the energy for fixing carbon dioxide from the conversion of ammonium to nitrite.