Nitrogen Cycle in Aquariums: Worksheet & Model
advertisement

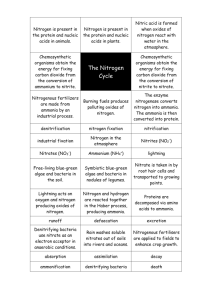
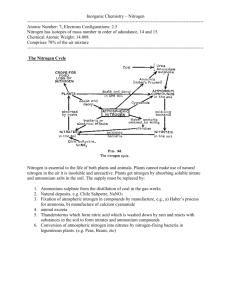
Nitrogen Cycle The element nitrogen, in different forms, cycles through the earth’s atmosphere, the land and aquatic systems. Within an aquarium ecosystem, nitrogen in can be both helpful to some organisms and harmful to others. In this exercise you will model the change in the nitrogen chemical formula and structure as it flows through the aquatic system. As a natural part of metabolism, fish excrete waste in a solid and a liquid form. This waste, mostly liquid, is in the form of ammonia (NH3). Ammonification also occurs at this time. Bacteria that is naturally occurring in the water, breaks down uneaten food, dead and decaying animals and plants into ammonia. When this ammonia is mixed with the water, it grabs another hydrogen from water molecules and forms ammonium (NH4+). The chemical equation for this is; NH3 + H2O <-> NH4++ OH-. Ammonia is extremely toxic to fish where ammonium is relatively harmless. In a small aquarium, the toxicity level of ammonia can occur quickly. The temperature and pH of the water determines which form the nitrogen will be in. The higher the temperature and pH, more of the nitrogen will be in the toxic ammonia form. It is at this part of the cycle that aquatic plants are most useful. These plants prefer the ammonium form of nitrogen as a food source. If plants are not part of the ecosystem or there are not enough plants, then nitrification takes place. In this process, bacterium uses the ammonia as an energy source. One type of bacteria turns the ammonia into nitrite (NO2), which is toxic to other organisms. Another type of bacteria turns the nitrite into nitrate (NO3) a non-toxic form. Unless the aquarium is completely closed off from the air, atmospheric nitrogen also enters the cycle in the form of N2. Denitrification converts nitrate back to N2 gas that is released back to the atmosphere. Don’t forget, fish like to eat plants. A. First, list the chemical formula for each of the following. Next, using the molecular model kits, construct each formula and then draw the structure. Name Chemical Formula Chemical Structure Ammonia Ammonium Nitrite Nitrate B. Use the information about nitrogen to create (as a group) the nitrogen cycle within an aquarium environment. Make a poster, or other form of presentation instructed by your teacher, to share with the class.