Solutes vs Solvents Experiment
advertisement

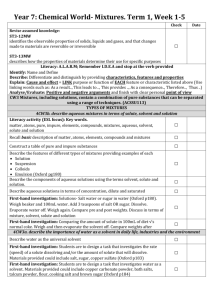
Exp. #2 Solutes vs. Solvents Experiment Date: ___________ Problem: What is a solution, solute, and a solvent? Hypothesis: Look at the substances that will be mixed with water. In the space below write which mixtures you think will have solutes and solvents and state why. Materials: orange juice vinegar sugar copper sulphate powdered milk rubber stopper graduated cylinder soup mix test tubes water Procedures: 1. Use a graduated cylinder to measure out 25 mL of room temperature water and pour it into each to the test tubes. 2. Mix 2 mL. of each of the following substances with the water in each of the 6 test tubes: orange juice vinegar sugar soup mix copper sulphate powdered milk 2. Put the rubber stopper over the end of the test tube and shake well for at least 30 seconds. 3. Allow each mixture to sit for a few minutes. 4. Record your results in the chart below. 5. Clean your equipment. Observations: Mixtures with Solutes and Solvents Chart Mixture with water Transparent Yes/No Floating or Suspended Material Yes/No Mechanical or Solution Solute or N/A Solvent or N/A Orange juice Soup mix Copper sulphate Powder milk Vinegar Sugar Conclusions: 1. Write a conclusion statement. 2. Which mixtures were solutions? How do you know? 3. Which mixtures were mechanical mixtures? How do you know? 4. Why did you write N/A for some mixtures? 5. Write a definition for solute, solvent, and solution.