8 - EXTRANET - Hospital Universitario Cruces
advertisement
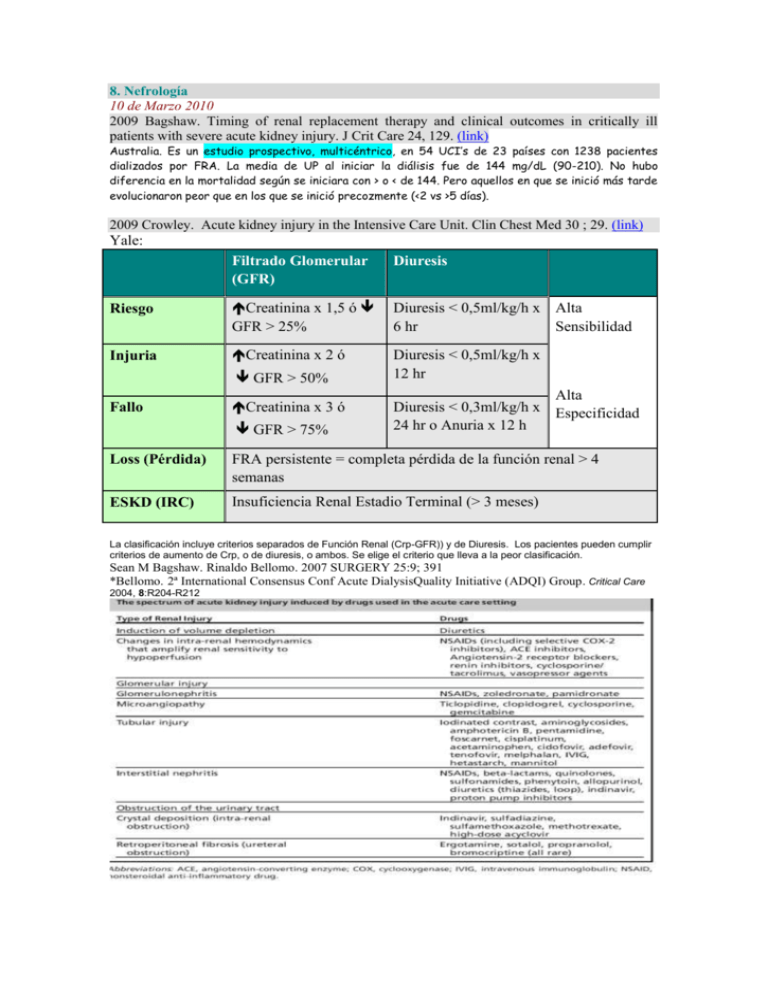
8. Nefrología 10 de Marzo 2010 2009 Bagshaw. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care 24, 129. (link) Australia. Es un estudio prospectivo, multicéntrico, en 54 UCI’s de 23 países con 1238 pacientes dializados por FRA. La media de UP al iniciar la diálisis fue de 144 mg/dL (90-210). No hubo diferencia en la mortalidad según se iniciara con > o < de 144. Pero aquellos en que se inició más tarde evolucionaron peor que en los que se inició precozmente (<2 vs >5 días). 2009 Crowley. Acute kidney injury in the Intensive Care Unit. Clin Chest Med 30 ; 29. (link) Yale: Filtrado Glomerular (GFR) Diuresis Riesgo Creatinina x 1,5 ó GFR > 25% Diuresis < 0,5ml/kg/h x Alta 6 hr Sensibilidad Injuria Creatinina x 2 ó Diuresis < 0,5ml/kg/h x 12 hr GFR > 50% Fallo Creatinina x 3 ó GFR > 75% Alta Diuresis < 0,3ml/kg/h x Especificidad 24 hr o Anuria x 12 h Loss (Pérdida) FRA persistente = completa pérdida de la función renal > 4 semanas ESKD (IRC) Insuficiencia Renal Estadio Terminal (> 3 meses) La clasificación incluye criterios separados de Función Renal (Crp-GFR)) y de Diuresis. Los pacientes pueden cumplir criterios de aumento de Crp, o de diuresis, o ambos. Se elige el criterio que lleva a la peor clasificación. Sean M Bagshaw. Rinaldo Bellomo. 2007 SURGERY 25:9; 391 *Bellomo. 2ª International Consensus Conf Acute DialysisQuality Initiative (ADQI) Group. Critical Care 2004, 8:R204-R212 Diálisis: En US se sigue utilizando más la HD intermitente que la continua (57 vs 36%) estudio PICARD, en el resto del mundo lo contrario (17 vs 80% en el estudio BEST-kidney). Si parece que la HD continua produce menos inestabilidad hemodinámica, pero no se asocia a mejora en la mortalidad (estudio HEMODIASAFE del 2006, PICARD del 2004 y previos). A lo mejor los estudios no han sido muy bien hechos, pero tb se ha señalado que la HD intermitente ha mejorado sus técnicas y que aunque es cierto que la HD continua depura citokinas, quizás no es muy relevante porque se produce más de lo que se depura. Se estudia la hemofiltración con alto volumen como un medio de “lavar” más citokinas: está en curso el estudio IVOIRE que busca comparar la mortalidad a 28 días entre el volumen de filtración standard (35 mL/kg/h) versus alto-volumen (70 mL/kg/h). Hay un estudio piloto con “super high-flux/high molecular weight cutoff (HCO) HD intermitente vs standard highflux (HF) IHD. La Hemofiltración “acelerada” tb está en estudio: se hace HDF intermitente con altos volúmenes de filtración comprimidos en menos horas (p ej 36 L en 9h). Hay tb modalidades “híbridas” que combinan algo de las diversas técnicas y que probablemente sean el futuro. Está en curso un estudio multicéntrico el “Dose Response Multicenter International (Do-Re-Mi)” para optimizar la práctica de las terapias continuas. The Acute Renal Failure Trial Network (ATN) Study. the Randomized Evaluation of Normal versus Augmented Level of renal replacement therapy in ICU (RENAL) Study (NCT 00221013), a large multicenter RCT being conducted in Australia of CVVHDF in severe AKI in ICU comparing 90-day mortality of patients treated with an ‘‘augmented’’ effluent flow rate of40 mL/kg/h versus patients treated with a ‘‘normal’’ flow rate of 25 mL/kg/h. Another multicenter trial, the IVOIRE study (NCT 00241228), is under way in France…. Too much for me…. Dialysis Dose: More recently, instead of prescribing RRT to achieve an absolute BUN level, quantification of dialysis dose has used the concept of urea clearance, exported from the field of chronic hemodialysis. For IHD it is described by a unit-less parameter: KT/Vurea Surprisingly, despite the body of trials examining dose of RRT support, all survey and observational studies of RRT support published to date convey quite clearly that the concept of ‘‘dose’’ in RRT in the ICU has been largely ignored or at least not fully adopted by practicing clinicians. Reluctance to adopt any targeted ‘‘dosing’’ of RRT may stem from the belief that toxicity of AKI is only partially related to small solute clearance, and thus prescription focused only on its modification should not be expected to yield substantial improvement in outcomes. Urea clearance, regardless of how great it is or by which modality of renal support it is achieved, tells us nothing about middle molecular weight or cytokine clearance or other aspects of the uremic state that may also be important in influencing outcomes. Bioartificial Kidney Currently available RRT is essentially designed to facilitate solute and water clearance. This approach hardly mimics the expansive metabolic and endocrinological functions of the human kidney and may explain the limited impact that RRT has had on survival in patients with AKI. A visionary strategy toward the treatment of AKI that addresses these previously overlooked functions of the kidney is the bioartificial kidney. Also known as the renal tubular assist device (RAD), the bioartificial kidney consists of a hemofiltration cartridge lined with nonautologous human renal tubule cells along the inner surface of hollow fibers within the device. Preclinical studies of the device showed that the cells within were able to perform renal transport and endocrine and metabolic functions…. 2009 Herrera. Técnicas de reemplazo renal continuas frente a las intermitentes: pro-continuas. Med Intensiva.;33(2):88. (link) (español) Carlos Haya: Málaga. Bagshaw con 9 estudios controlados en 1.403 pacientes, una vez más, no muestra diferencias en la mortalidad (odds ratio [OR] =0,99; IC del 95%, 0,78-1,26) o en la recuperación renal (OR = 0,76; IC del 95%, 0,28-2,07). Por lo tanto, según estos datos, no hay evidencia a favor de ninguna modalidad. Como Bagshaw muestra en su análisis, estos estudios aportan resultados dispares, ninguno de ellos reúne todos los requisitos de calidad exigibles y todos ellos presentaron algún problema metodológico. ¿POR QUÉ USAR ENTONCES LAS TCDE?: a) su uso durante 24 h al día permite la extracción de volúmenes muy elevados de fluido sin repercusión para el paciente y se acompaña de una mayor tolerancia hemodinámica y respiratoria al tratamiento; b) el uso de convección podría repercutir en la evolución de la sepsis grave, y c) no requieren de infraestructura ni personal especial, lo que facilita su uso fuera de la unidad de diálisis a cualquier hora del día. Definir actualmente qué técnica es de elección es una tarea casi imposible. Un estudio multicéntrico internacional actualmente en marcha (DOREMI), en poco tiempo, podría proporcionarnos una información más detallada sobre cómo elegir y prescribir las TDE. En el momento actual, tan sólo podemos asegurar que no hay evidencia a favor o en contra de ninguna de las modalidades de tratamiento, pero que los datos disponibles apuntan a que, al menos en determinadas circunstancias (pacientes ingresados en UCI y, sobre todo, en hospitales sin servicios de diálisis, pacientes inestables las TCDE proporcionan ventajas por su mayor capacidad para eliminar fluidos, su tolerancia hemodinámica, actúan sobre los mecanismos fisiopatológicos del SRIS y necesitan < infraestructura. 2009 Barrio: Técnicas de reemplazo renal continuas frente a las intermitentes: pro-intermitentes Med Intensiva.;33(2):93 (link) (español) CONCLUSION: Las técnicas continuas de TRS, de forma significativa, conllevan una mejor tolerancia hemodinámica, pero a costa de un riesgo significativamente mayor de coagulación recurrente del filtro de diálisis. Ambas técnicas presentan una mortalidad similar y, asimismo, las expectativas de recuperación de la función renal son equivalentes con ambas modalidades de TRS. Por lo tanto, la elección de una u otra deberá individualizarse en función de la situación particular de cada paciente y de la disponibilidad local de estas técnicas. 11 de marzo de 2010 2009 Kishen. Standards and Recommendations for the Provision of Renal Replacement Therapy on Intensive Care Units in the UK. (link) 3.1 Indications for starting therapy Classical ‘renal’ indications for starting renal replacement therapy (RRT) are: • Rapidly rising serum urea and creatinine or the development of uraemic complications • Hyperkalaemia unresponsive to medical management • Severe metabolic acidosis • Diuretic resistant pulmonary oedema • Oliguria or anuria ‘Non renal’ indications for starting RRT are: • Management of fluid balance e.g. in cardiac failure • Clearing of ingested toxins • Correction of electrolyte abnormalities • Temperature control • Removal of inflammatory mediators in sepsis 3.2 Timing of therapy There is increasing evidence that early initiation of RRT is beneficial and may have survival or kidney function recovery advantages 3.3 Standard/recommendation 1. Conventional starting criteria for RRT should be used (Grade D). 2. Treatment should be started before complications develop (Grade E). 3. The rate of change of urea and creatinine is more significant than their absolute levels (Grade C), however in most cases RRT should be started before urea is 20 – 30 mmol/L. In many circumstances, starting RRT well before urea rises to these levels may well be justified. 4. Initiation of RRT on the basis of fluid balance, urine output, potassium level or degree of acidosis will be dependent on the patient’s clinical condition. 4 Intermittent versus continuous therapies 4.1 Current practice • In the UK, Western Europe and Australia, continuous therapies are the predominant mode of delivery of RRT • More than 10,000 patients are provided with RRT in UK ICUs annually • About 90% of all ICUs in the UK provide RRT, most using CVVH or CVVHDF (18) • Worldwide, 80% of patients who need RRT for AKI on the ICU are treated with continuous therapies, 16.9% of patients are treated with intermittent therapies and 3.2% receive either peritoneal dialysis or slow continuous ultrafiltration 4.5 Development of hybrid therapies Given both intermittent and continuous therapies have their own potential benefits, hybrid therapies such as slow low efficiency daily dialysis (SLED(D)) have been developed as a ‘best of both worlds’. 4.7 Standard/recommendation 1. There is insufficient evidence to recommend CRRT over IHD or vice versa in terms of patient mortality, but data tend to suggest a better renal outcome with CRRT. 2. Consensus favours CRRT for patients on the ICU with AKI (Grade E). In particular it appears appropriate to use CRRT in haemodynamically unstable patients and those with fluid balance issues. 3. CRRT also appears to offer particular benefit in patients with, or at risk of cerebral oedema ( C). 4. IHD is appropriate to use in haemodynamically stable patients during their recovery from a critical illness. Its use will be determined by local organisational factors. 5. PD should not be used routinely for critically ill patients with AKI on the ICU. (Grade C). 5.1 Principles of solute removal • Different methods of RRT are in part differentiated by the predominant method of solute removal, diffusion or convection • Diffusion is the movement of solutes down a concentration gradient across a semi permeable membrane • With membranes with high hydraulic permeability (high permeability to water), plasma water moves across the membrane carrying solutes with it (solvent drag). This bulk-flow of solutes is termed convection or mass convective transfer. Water movement and therefore convection can be enhanced if pressure is applied across the membrane • The predominant method of solute removal for each therapy is: • Intermittent haemodialysis (IHD) – diffusion • Continuous veno-venous haemofiltration (CVVH) - convection • Continuous veno-venous haemodialysis (CVVHD) - diffusion • Continuous veno-venous haemodiafiltration (CVVHDF) – diffusion and convection There are several ways to determine the efficiency of any renal replacement therapy, one such method widely used in ESKD is Kt/V. This is the fractional clearance of a given solute (usually urea), which takes into consideration therapy duration (t) and the volume of distribution of the marker molecule in the body (V). Early work suggested that patients with AKI on the ICU with an intermediate severity of illness did better with a higher dose of dialysis, delivering a Kt/V of greater that 1.0 (42). A more recent study showed that a higher intensity of IHD improved outcome with a delivered Kt/V of 0.92 based on 6.2 treatments per week, with a mean of 3.3 hour sessions 5.6 Standard/recommendation 1. For IHD a minimum Kt/V of 1.2 should be delivered 3 times a week in patients with kidney failure (Grade A). There is increasing evidence that an increase to daily or near daily IHD may be more beneficial in critically ill patients with AKI but further information from trials is awaited. 2. The ideal dose for CRRT is not known; however 35 ml/kg/h of ultrafiltrate production is recommended as a minimum for CVVH (post-dilution) and CVVHDF (Grade C). Results from RENAL study (52) are awaited to increase our understanding. Besides, 35 ml/kg/h ensures that adequate dose of CRRT is delivered despite filter down times. 3. Pre-dilution CRRT reduces solute clearance and an increase of 15% for ultrafiltration rates of 2 L/h and up to 40% for rates of 4.5L/h or more should be considered. 4. There is no evidence to suggest that CVVH (convection) is superior to CVVHDF (convection plus diffusion) in terms of patient outcome or renal outcome (or vice versa). If adequate ultrafiltration rates cannot be achieved using CVVH due to machine limitations, then CVVHDF should be considered. A predominantly convective mode of clearance may be considered in severe sepsis (see section 12). 5. Assessment of solute clearance on CRRT/IHD should be as per local unit policy or by clinician direction. Urea and creatinine should be measured daily, but there is no recommendation as to the absolute level that should be achieved. A normal pH and potassium should be achieved and arterial blood gas samples should be analysed at a frequency dictated by the clinical state of the patient. 6. Efforts should be made to maintain effective therapy, with at least 85%of the prescribed dose being delivered. (Grade E). 6 Choice of replacement fluid 6.4 Standard/recommendation 1. Bicarbonate-based fluids may have theoretical disadvantages but currently there are no data favouring either kind as replacement fluid (Grade C). 2. A rise of lactate of greater or equal to 5 mmol/L (from base-line) associated with a worsening metabolic acidosis suggests lactate-intolerance, under these circumstances the buffer should be changed to bicarbonate. Initial use of bicarbonate is recommended for patients with a severe preexisting lactic acidosis pH <7.2 with associated lactate of ≥ 8 mmol/L or a lactate rise of ≥5 mmol/L with a drop in pH i.e., lactate intolerance or with severe liver dysfunction (Grade C). 7 Choice of dialysis/filter membrane 1. There is no conclusive evidence to suggest that modified cellulose membranes should be avoided, however until proven otherwise, biocompatible synthetic membranes are recommended over cellulose based membranes 8 Choice of anticoagulation 8.12 Standard/recommendation 1. Consensus exists that anti coagulation free CRRT can be successfully achieved (Grade D). No anticoagulation is suggested when any of the following are present: a. INR > 2-2.5 b. APTT > 60 seconds c. Thrombocytopenia e.g. platelet count < 60 x 103/mm3 d. High risk of bleeding e. When patients are receiving activated protein C 2. In patients who have a normal coagulation profile, normal platelet count and who are not at risk of bleeding, UFH or LMWHs can be used (Grade C). UFH is inexpensive, familiar to all clinicians and has an antidote. 3. Suggested use of UFH: Optional loading dose of 2000-5000 IU depending on the clinical situation. Loading dose may be omitted in patients at increased risk of bleeding. Infusion of 5-10 IU/kg/h. APTT should be checked 6 hours after starting and then regularly until stable aiming for an APTT of 1-1.4 times normal (Grade E). A higher level of anticoagulation can be considered if clinically indicated. 4. The APTT does not always reflect the anticoagulation effect of UFH and there is no correlation between increasing APTT and filter life. 5. ACT is imprecise, especially at low doses of UFH and should be interpreted with caution. Its use in the critically ill to monitor coagulation is not recommended. 6. Reference should be made to individual drug guidelines when using LMWHs, but for prolonged use monitoring of anti-Xa levels is recommended (target 0.25-0.35 U/ml) (Grade E). 7. All patients being treated with UFH or LMWHs should have daily platelet counts performed. In the event of the development of heparin HIT, all heparins should be stopped and an alternative method of coagulation considered. (Grade C). 8. There is no evidence to suggest newer heparin alternative such as danaparoid, hirudin, fondaparinux or argatroban are better than UFH/LMWHs. There are still only limited safety data and therefore no recommendations can be made. 9. Regional citrate anticoagulation is an effective therapy, particularly if there is an increased risk of bleeding (Grade C). To avoid complications a strict protocol should be developed and adhered to. 10. Prostaglandins are also effective ‘anticoagulants’ if there is a high risk of bleeding. (Grade E). A low dose is generally used (2.5 – 10 ng/kg/min). They may be especially useful in complex situations e.g., AKI requiring RRT in a patient with recent subarachnoid haemorrhage. 11. Pre-dilution can be used as an adjunct to anticoagulation (Grade C), and is highly recommended if no anticoagulation is to be used. To preserve efficiency, ultrafiltrate volumes need to be increased accordingly. 9 Vascular access 9.4 Standard/recommendation 1. A polyurethane, > 11FG dual lumen venous catheter should be used (Grade D). 2. Right internal jugular lines are associated with the least recirculation (Grade C) however the site of insertion will be guided by patient factors. In patients with a high chance of remaining dialysis dependant, the subclavian route should be avoided if possible (grade C) as this site has a high rate of vessel stenosis. For subclavian and jugular lines the tip should sit in the superior vena cava, and for the femoral, the tip should sit in the inferior vena cava, this often necessitates the use of a line of 20-24cm in length. 3. A vessel ultrasound should be used for insertion (Grade B). 4. Tunnelled access lines may be considered in patients who are deemed sepsis free and awaiting transfer to a renal unit for ongoing renal replacement therapy. 5. Each unit should develop guidelines for the insertion of lines, ongoing care of the lines and appropriate microbiological surveillance. 10 Drug dosing during renal replacement therapy 10.3 Standard/recommendation 1. Drug dosing during CRRT should not be based on ‘nomograms’ prepared for chronic stable patients or patients on IHD. 2. Drug levels should be measured if possible. Clinicians should be careful not to under-dose these patients, particularly if using antibiotics to treat severe sepsis. 3. Access to a pharmacist with expertise in drug dosing during RRT is advisable but it is suggested that each unit has easily accessible or ‘bedside’ dosing guidelines for commonly prescribed drugs for patients who are on RRT. 12 Non-renal indications for RRT and allied modalities 12.6 Standard/recommendation 1. Many non-renal indications of CRRT are emerging but there needs to be more evidence before routine use in other clinical situations is recommended. 2. CRRT can be used to remove drugs or toxins however IHD will provide higher clearance rates. If IHD cannot be used due to availability or haemodynamic instability, CVVHDF or CVVH with higher blood flow and ultrafiltration rates can be used. 3. The use of haemofiltration, therapeutic plasma exchange or absorptive therapies as adjunctive treatments in severe sepsis have biological rationale (Grade D). Ultrafiltrate volumes of < 2L/h in adults are unlikely to provide benefit (Grade C), 35 ml/kg/h of ultrafiltrate production appears to be the minimum effective dose(C)but higher doses may be needed. 4. If RRT is needed for AKI in a patient who also has severe sepsis, 35 ml/kg/h should be the minimum (Grade C). Initial studies are promising but further studies are needed before HVHF can be recommended for widespread use in cases of severe septic shock. 5. CVVH is better than PD for the treatment of AKI in septic patients (Grade C), and consensus is that CRRT is better than IHD for haemodynamically unstable septic patients (Grade E). 6. Ultrafiltration, with or without haemofiltration can be used to manage fluid overload, in particular in patients with severe cardiac failure. 2009 Palazzo. Sodium bicarbonated the bicarbonate challenge test in metabolic acidosis:A practical consideration. Current Anaest & Critical Care 20; 259. (link) - La indicación más común para la administración de bicarbonato es la acidosis metabólica con pH <7,1, cuando es poco probable que se normalice espontáneamente en poco tiempo, por ejemplo en situaciones de insuficiencia renal aguda o isquemia aguda de un órgano principal. - El bicarbonato es tb usado ocasionalmente para diuresis forzada alcalina, para promover la excreción de drogas por ej en la acidosis metabólica después de una sobredosis de salicilato: típicamente se administra 1 L de bicarbonato de sodio isotónico (1,26%) , en la primera hora, seguido por 500 ml de dextrosa al 5%, + 500 ml de solución salina + 500 ml de bicarbonato de sodio 1,26% en intervalos de una hora. La Alcalinización también aumenta la excreción de barbitúricos. - En la rabdomiolisis. La Alcalinización suprime la reactividad de la mioglobina libre y por consiguiente reduce la generación de isoprostanos. Se suele dar bicarbonato y Manitol para obtener un pH urinario de 6 cuando la CK es > 5000 UI/L. Puede producirse hipocalcemia. La acidosis metabólica asociados con hipoperfusión S. cardiogénico, hipovolémico (pérdida de sangre) o por secuestro (sepsis), debe ser corregida de manera agresiva con medidas de reanimación antes de cualquier consideración de utilizar bicarbonato. - La cetoacidosis diabética, en particular, por lo general no necesita de bicarbonato a menos que el pH es <7,0, y sólo durante un breve período hasta que el pH eleva por encima de 7,0. Una vez que el volumen circulante ha sido restaurado y la insulina se ha introducido, los cetoácidos y el lactato se convierten en bicarbonato en el hígado. . El bicarbonato se puede utilizar como parte de una serie de medidas para reducir temporalmente la hiperpotasemia. Peligros: Hipopotasemia, Hipocalcemia, Hipercarbia, Acidosis paradójica intracelular (parece que es menos de lo que se pensaba) - 2009 Palevsky. Renal Support in Acute Kidney Injury — How Much Is Enough?. N Engl J Med 361;17 . 1699 (link) Editorial sobre el estudio RENAL: dice que los resultados son similares a los del estudio “Acute Renal Failure Trial Network Study”, y que no es que la intensidad de la terapia de reemplazamiento no sea importante, si no que no debe tomarse como un parámetro inamovible, y que debe individualizarse según las características del paciente. 2009 Payen. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: A randomized controlled trial. Crit Care Med; 37:803. (link) 80 pacientes con sepsis fueron reclutados en las primeras 24 horas de presentar 1 fallo orgánico. Intervenciones: Los pacientes fueron asignados aleatoriamente al grupo 1 (HF), que recibió hemofiltración (25 ml/kg /hr) durante un periodo de 96 horas, o al grupo 2 (C) que fueron manejados de manera convencional. El estudio se suspendió después de que un análisis provisional con 76 RND mostrara que el número y la gravedad de los fallos orgánicos eran significativamente mayor en el grupo de HF (p <0,05). No pudo detectarse diferencia en los niveles de citokinas entre ambos grupos. Conclusión: Estos datos sugieren que la pronta aplicación de la hemofiltración venovenosa continua es perjudicial en sepsis graves y shock séptico. Este estudio no excluye el efecto de la hemofiltración de alto volumen (> 35 ml / kg / h) en el curso de la sepsis, que podría tener resultados diferentes. 2009 The RENAL Replacement Therapy Study Investigators . Intensity of Continuous RenalReplacement Therapy in Critically Ill Patients. N Engl J Med;361:1627. (link) Bellomo. Randomized Evaluation of Normal versus Augmented Level (RENAL) Replacement Therapy Study. 1508 pacientes con FRA en UCI asignados aleatoriamente a recibir hemodiafiltración venovenosa continua a un caudal de efluente total de 25 vs 40 ml/Kg/h. El estudio continuó hasta la recuperación de la función renal, o hasta que el paciente era dado de Alta de UCI. En ambos grupos de tratamiento, el 44,7% de los pacientes murió en los primeros 90 días. (odds ratio, 1,00; intervalo de confianza 95%: 0,81 a 1,23 P=0.99. El 94,4% de los pacientes que estaban vivos a los 90 días no precisaron más diálisis. A los 90 días, 6.8% de los sobrevivientes en el grupo de alta intensidad seguía precisando diálisis vs 4.4% en los sobrevivientes del grupo de baja (odds ratio, 1.59; 95% CI, 0.86 to 2.92; P = 0.14). La Hypophosphatemia fue mas frecuente en el grupo de alta intensidad (65% vs. 54%, P<0.001).











