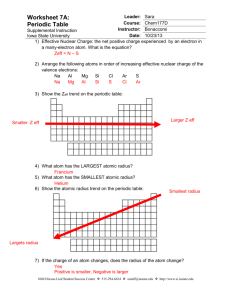
Zeff & Periodic Trends Worksheet: Chemistry
advertisement
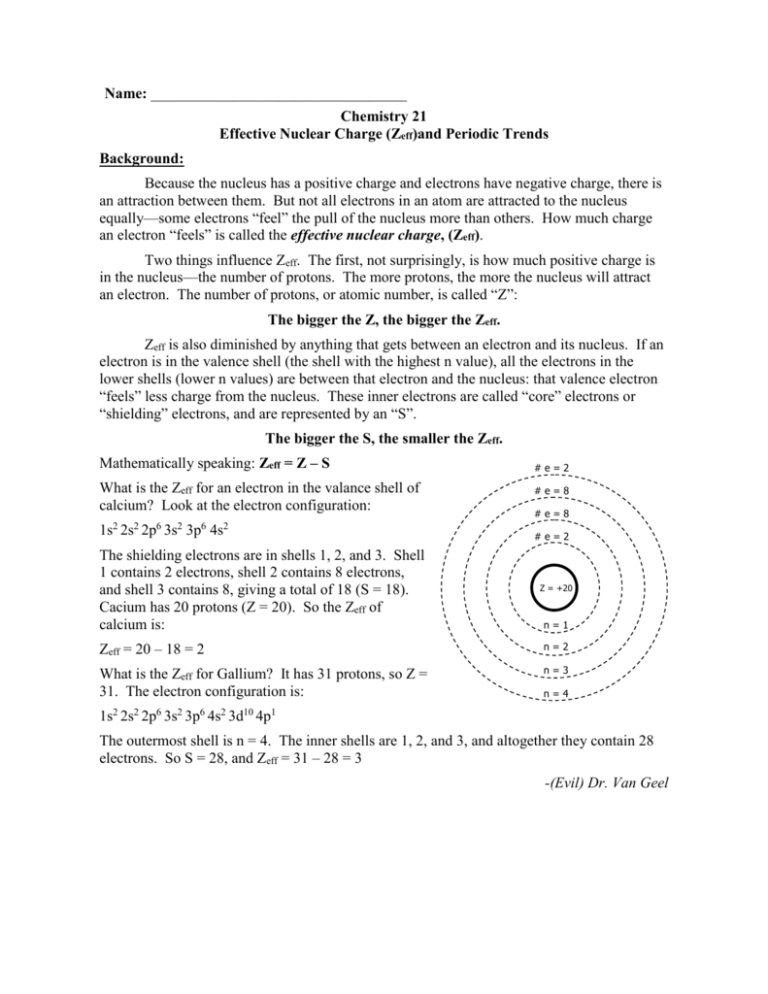
Name: __________________________________ Chemistry 21 Effective Nuclear Charge (Zeff)and Periodic Trends Background: Because the nucleus has a positive charge and electrons have negative charge, there is an attraction between them. But not all electrons in an atom are attracted to the nucleus equally—some electrons “feel” the pull of the nucleus more than others. How much charge an electron “feels” is called the effective nuclear charge, (Zeff). Two things influence Zeff. The first, not surprisingly, is how much positive charge is in the nucleus—the number of protons. The more protons, the more the nucleus will attract an electron. The number of protons, or atomic number, is called “Z”: The bigger the Z, the bigger the Zeff. Zeff is also diminished by anything that gets between an electron and its nucleus. If an electron is in the valence shell (the shell with the highest n value), all the electrons in the lower shells (lower n values) are between that electron and the nucleus: that valence electron “feels” less charge from the nucleus. These inner electrons are called “core” electrons or “shielding” electrons, and are represented by an “S”. The bigger the S, the smaller the Zeff. Mathematically speaking: Zeff = Z – S What is the Zeff for an electron in the valance shell of calcium? Look at the electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 #e=2 #e=8 #e=8 #e=2 The shielding electrons are in shells 1, 2, and 3. Shell 1 contains 2 electrons, shell 2 contains 8 electrons, and shell 3 contains 8, giving a total of 18 (S = 18). Cacium has 20 protons (Z = 20). So the Zeff of calcium is: Z = +20 n=1 Zeff = 20 – 18 = 2 n=2 What is the Zeff for Gallium? It has 31 protons, so Z = 31. The electron configuration is: n=3 2 2 6 2 6 2 10 n=4 1 1s 2s 2p 3s 3p 4s 3d 4p The outermost shell is n = 4. The inner shells are 1, 2, and 3, and altogether they contain 28 electrons. So S = 28, and Zeff = 31 – 28 = 3 -(Evil) Dr. Van Geel Procedure: Open the Excel file “Periodic Trends Data.xls” in the documents section of the course website. Click on the Effective Nuclear Charge tab. You will see an abridged periodic table; it has only the “main group elements”; all the elements except the transition and inner transition metals. Next to it there is a blank version of the table. Calculate the Zeff for all the elements and enter them into the table (this won’t take nearly as long as you think once you recognize the pattern). 1. Using the graph wizard in Excel, make a line graph of the values in Period 2. Does Zeff increase, decrease, or remain the same as you move from left to right across a period? __________________________ 2. Make a line graph of the values in group 1. Does Zeff increase, decrease, or remain the same as you move down a group? ______________________________ Knowing the pattern of Zeff is very important! The values of Zeff explain a lot of the other trends you are about to discover. Atomic Radius The atomic radius is the distance from the nucleus to the valence electrons measured in Angstroms (1 Å = 1 x 10-10 m). Click on the Atomic Radius tab. You will see an abridged periodic table and a table with atomic radius values. 1. Make a line graph of the values in period 4. Does the radius increase, decrease, or remain the same as you move from left to right across a period? __________ 2. What explains this trend? (Hint: think about Zeff.) ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 3. Make a line graph of the values in group 18. Does the radius increase, decrease, or remain the same as you move down a group? ______________________ 4. Can the trend in Zeff explain the trend of the radii within a group? Why or why not ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 5. What explains the trend within a group? ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 6. On the periodic table shown, draw an arrow to represent the overall trend in atomic radii (the end of the arrow is where the radii are smallest, the point is where they are largest). 8. If an atom forms a positive ion by losing an electron, do you think the radius of the ion would be bigger or smaller than the neutral atom? Explain. ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 9. If an atom forms a negative ion by gaining an electron, do you think the radius of the ion would be bigger or smaller than the neutral atom? Explain. ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ Ionization Energy Ionization energy is the amount of energy needed to remove a valence electron from an atom. This is a measure of how strongly the nucleus holds on to the outer electrons. 10. Click on the ionization energy tab. Make a line graph of the values in period 3. Does the ionization energy increase, decrease, or remain the same as you move from left to right across a period? ____________________________ 11. What explains the trend within a period? ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 12. Make a line graph of the values in group 17. Does the ionization energy increase, decrease, or remain the same as you move down a group? ______________ 13. What explains the trend within a group? (Hint: it has something to do with atomic radius.) ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 14. On the periodic table shown, draw an arrow to represent the overall trend in ionization energy (the end of the arrow is where the ionization energy is lowest smallest, the point is where it is greatest). Electronegativity Electronegativity is a measure of how much an atom “hogs” electrons when it is bound to other atoms with a chemical bond. The more electronegative the atom, the more it will hog the electrons. 15. Click on the electronegativity tab. Make a line graph of the values in Period 5. Does the electronegativity increase, decrease, or remain the same as you move from left to right across a period? ___________________________ 16. What explains the trend within a period? ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 17. Make a line graph of the values in group 2. Does the electronegativity increase, decrease, or remain the same as you move down a group? ________________ 18. What explains the trend within a group? ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 19. On the periodic table below, draw an arrow to represent the overall trend in electronegativity (the end of the arrow is where the electronegativity is lowest, the point is where it is highest). Follow-up Questions: 1. Different elements are more reactive than others; some are more likely to undergo chemical reactions than others. When metals react, they almost always lose electrons. The easier it is for the metal to lose electrons, the more reactive the metal. What do you think is the most reactive metal, and why? ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 2. Conversely, the more reactive the nonmetal, the stronger it holds on to its electrons. What do you think is the most reactive nonmetal, and why? (Remember that noble gases are not reactive at all.) ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 3. Circle the element from each set with the largest atomic radius. Explain your reasoning. Sr Ti Ra Li ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ F Al In As ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 4. Circle the element from each set with the smallest ionization energy. Explain your reasoning. Tl Po Se Ga ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 5. Circle the ion with the larger radius. Explain your reasoning. Na+ F- ________________________________________________________________ ________________________________________________________________ ________________________________________________________________