File
advertisement

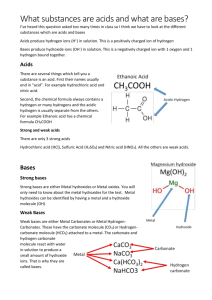
Standard Grade Chemistry Summary Notes Topic 9 : Reactions Of Acids General Learning Outcomes Neutralisation is the reaction of acids with neutralisers. Everyday examples of neutralisation : Reducing acidity in soil by adding lime The use of lime to reduce acidity in lakes caused by acid rain Treatment of acid indigestion Neutralisation moves the pH of an acid toward 7. The reaction of acids with alkalis is an example of neutralisation. Neutralisation moves the pH of an alkali toward 7. The reaction of H+(aq) to form water is an example of neutralisation. Neutralisation of dilute hydrochloric acid forms chloride salts. Neutralisation of dilute nitric acid forms nitrate salts. Neutralisation of dilute sulphuric acid forms sulphate salts. An acid reacts with a metal carbonate to give off carbon dioxide. Acid rain erodes buildings and carbonate rocks. Acids react with some metals to form hydrogen gas. In the reaction hydrogen ions form hydrogen molecules. When dilute hydrochloric acid reacts with a metal, the metal chloride and hydrogen gas are formed. When dilute sulphuric acid reacts with a metal, the metal sulphate and hydrogen gas are formed. Acid rains corrodes (rusts) structures made from iron. Precipitation is the reaction of two solutions to form an insoluble product called a precipitate. You must be able to name the insoluble product formed by the reaction of two solutions. Credit Learning Outcomes The reaction of hydrogen ions and hydroxide ions form water. A salt is a substance in which the hydrogen ion of an acid has been replaced by a metal ion (or the ammonium ion) The reaction of hydrogen ions and carbonate ions forms water and carbon dioxide. In the preparation of a given salt it is often easier to use an insoluble metal carbonate or metal oxide as the neutraliser as excess/ unreacted neutraliser is easy to remove by filtration. A base is a substance which neutralises an acid. Bases which dissolve in water form alkalis. Insoluble salts can be formed by precipitation. You must be able to carry out calculations to find the concentration of acids/ alkalis from volumetric titration What Happens In A Neutralisation Reaction (General) Neutralisation is the reaction of acids with neutralisers e.g. alkalis, metal (and ammonium) carbonates, metal oxides. In a neutralisation reaction the pH of the acid moves up towards 7. If an alkali is being used as the neutraliser, its pH moves down towards 7. New substances called salts and water are formed in neutralisation reactions. The name of the salt that is produced depends on the type of acid and the type of neutraliser being used. The reaction of hydrogen ions -H+ (aq) to form water is an example of neutralisation. Reactions Of Acids (General) The general word equations below describe what happens when acids react with neutralisers. alkali + acid salt + water metal oxide + acid salt + water metal carbonate + acid salt + water + carbon dioxide Naming The Products Of Neutralisation Reactions (General) Salts are made by the reaction of acids with a neutralisers. Examples of salts are sodium chloride, potassium nitrate, calcium sulphate. The first part of the name is the name of a metal. The second part of the name comes from the name of the acid. Acid hydrochloric sulphuric nitric Second part of name of salt formed chloride sulphate nitrate Example : alkali + acid salt + water sodium oxide + hydrochloric acid sodium chloride + water potassium hydroxide + nitric acid potassium nitrate + water Reactions Of Acid And Metals (General) Metals react with acids but hydrogen is made instead of water. The acidity of the acid decreases and the pH moves up towards 7. However this is not an example of neutralisation as water is not formed. The general word equation for the reaction is : metal + acid salt + hydrogen Example : zinc + sulphuric acid zinc sulphate + hydrogen Zn + H2SO4 ZnSO4 + H2 (Credit) Remember that at credit level you will be expected to balance formula equations. Ionic Equations (Credit) When acids and alkalis react, hydrogen ions and hydroxide ions join to form water. H+ (aq) + OH- (aq) H2O (l) When acids and metal carbonates react, hydrogen ions and carbonate ions react to form water and carbon dioxide. H+ (aq) + CO32- (aq) H2O (l) + CO2 (g) When acids react with metals, hydrogen ions form hydrogen molecules. 2H+(aq) + M(s) H2(g) + M2+ (aq) (M represents a metal in the above equation)