27. Any compound which when added to water breaks apart
advertisement

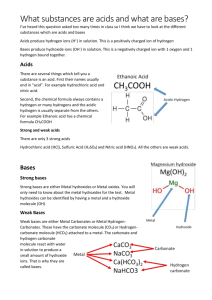
27. Any compound which when added to water breaks apart (dissociates) to produce a solution that contains hydrogen ions is called a(n) ACID. P.219 28. The hydrogen ions in an acid are sometimes called HYDRONIUM ions. P. 219 29. The abbreviation which is used to represent a hydrogen ion is H+. 30. The chemical formula for an acid always begins with the letter H. P. 219 P. 219 31. The general formula for an acid is H - NON METAL. 32. The more hydrogen ions that are in a solution the STRONGER the acid will be. P. 220 33. Acids have a(n) SOUR taste. (Example: Lemmon Juice) P. 214 34. Acids react with many metals to produce HYDROGEN gas. P. 213 35. Acids react with carbonates (compounds containing CO3) to form CARBON DIOXIDE gas and water. P. 214 36. Acids turn blue litmus paper RED. P. 213 37. Any compound which when added to water breaks apart (dissociates) to produce a solution that contains hydroxide ions is called a(n) BASE. P. 220 38. The hydroxide ions in a base are sometimes called HYDROXAL ions. 39. The abbreviation which is used to represent a hydroxide ion is OH-. P.220 40. The chemical formula for a base always ends in OH. P. 220 41. The general formula for a base is METAL - OH 42. The more hydroxide ions that are in a solution the STRONGER the base will be. P. 220 43. Bases have a(n) BITTER taste. (Example: Soap) P. 215 44. Bases feel SLIPPERY when you touch them. (Example: Soap) P. 215 45. Bases turn red litmus paper BLUE. P. 215 46. The strength of an acid or base is measured using the pH scale. P. 220 47. The abbreviation pH stands for POTENTIAL HYDROGEN. 48. The pH scale has a range of values from 0 to 14. P. 220 - 221 49. Any liquid which has a value on the pH scale from 0 to 6.9 is considered to be an acid. P. 220 - 221 50. The LOWER the pH value is for a liquid the stronger the acid will be. P. 220 - 221 51. Any liquid which has a value on the pH scale from 7.1 to 14 is considered to be a base. P. 220 - 221 52. The HIGHER the pH value is for a liquid the stronger the base will be. P. 220 - 221 53. Any liquid which has a value of 7 on the pH scale is considered to be neutral and is not an acid or a base. P. 220 54. The chemical formula for water is H2O or HOH. 55. Pure water is not an acid or a base because it is NEUTRAL and it has a pH of 7. P. 220 56. Any substance which changes color in the presence of an acid or a base is called a(n) INDICATOR. P. 214 57. Some common indicators which are used to determine whether a solution is an acid or base and possibly how strong of an acid or base it is are: P. 214, 216 A. BLUE AND RED LITMUS PAPER B. PHENOLPHTHALEIN C. RED CABBAGE JUICE D. BROM THYMOL BLUE 58. A base will neutralize an acid when they’re combined together to form a(n) SALT and WATER. P. 222 - 223 59. The general formula for the chemical reaction which occurs when a base neutralizes an acid is: P. 222 - 223 Example ACID + BASE SALT + WATER HCl Acid + NaOH Base NaCl Salt + H2O Water 60. The general formula for a salt is METAL – NON METAL. Example: Na – Cl Metal - Non- Metal