ISMP Medication Safety Alert - Institute For Safe Medication Practices
advertisement

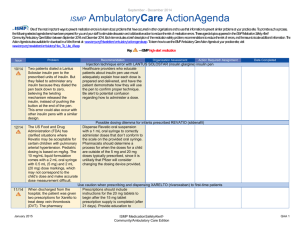
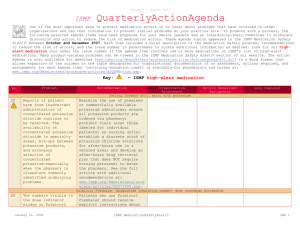
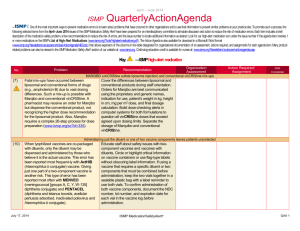
October – December 2013 ISMP QuarterlyActionAgenda One of the most important ways to prevent medication errors is to learn about problems that have occurred in other organizations and to use that information to prevent similar problems at your practice site. To promote such a process, the following selected items from the October-December 2013 issues of the ISMP Medication Safety Alert! have been prepared for an interdisciplinary committee to stimulate discussion and action to reduce the risk of medication errors. Each item includes a brief description of the medication safety problem, a few recommendations to reduce the risk of errors, and the issue number to locate additional information as desired. Look for our high-alert medication icon under the issue number if the agenda item involves one or more medications on the ISMP’s List of High-Alert Medications (www.ismp.org/Tools/highalertmedications.pdf). The Action Agenda is also available for download in a Microsoft Word format (www.ismp.org/Newsletters/acutecare/articles/ActionAgenda1401.doc) that allows expansion of the columns in the table designated for organizational documentation of an assessment, actions required, and assignments for each agenda item. Many product-related problems can also be viewed in the ISMP Medication Safety Alert! section of our website at: www.ismp.org. Continuing education credit is available for nurses at: www.ismp.org/Newsletters/acutecare/actionagendas.asp. Key: —ISMP high-alert medication Organization Assessment No. Problem (23) More than 1,100 patients received less potent chemotherapy than intended. Large bags of chemotherapy had been prepared and divided into smaller doses for multiple patients. Overfill in the large bags was not considered when listing the concentration on the label because the compounding pharmacy thought each large bag was to be used as a single dose. Although the full dose in each bag was listed on the label, the actual concentration on the label was incorrect. There are several methods that can be used to prepare sterile products, each with specific means for managing the overfill volume to avoid confusion. Understanding and managing IV container overfill Choose the most appropriate method of preparing each medication infusion according to whether or not the volume/concentration is critical. Obtain a list of overfill amounts of commonly used products from vendors for reference as necessary. For continuous infusions titrated to effect, ensure standardization in the preparation process in order to avoid variations in concentration and inconsistencies with the dose delivered. For a single dose drug infusion, the most critical aspect of the process is ensuring that the entire contents in the container are administered; the label should include a reminder, “Infuse entire contents for full dose.” (22) As the use of U-500 insulin grows, so do the number of errors, mostly related to dosing confusion caused by not having a syringe with a U-500 scale. Healthcare providers and patients rely on syringes meant for U-100 insulin to measure U-500 insulin doses. This results in communicating the dose by the number of units that correspond to the U-100 syringe. Another source of confusion is name similarity since HUMULIN R is the name used for both U-100 insulin and U-500 insulin. Safety concerns surrounding the use of U-500 insulin Until U-500 syringes or pens are available, use tuberculin syringes to measure doses by volume, using a dosing conversion chart (available at: www.ismp.org/sc?id=260). Total doses should be expressed in both units and volume (i.e., 200 units [0.4 mL]). To minimize name confusion, ensure the strength is listed with each HUMULIN R insulin product during order entry. Separate U-100 insulin and U-500 insulin vials in storage areas. January 30, 2014 Recommendation ISMP MedicationSafetyAlert! Action Required/ Assignment Date Completed QAA 1 October - December 2013 ISMP Problem No. (23) (25) QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed Initiative to eliminate tubing misconnections Catheter misconnections happen when tubing A phased-in approach to launch the new from one type of delivery system is connected connectors, starting with enteral devices, will to another delivery system that serves a occur in 2014. Organizations should review different function. An international effort is the publication, Stay Connected, for underway to standardize the various types of Frequently Asked Questions connectors used in healthcare, making them (www.ismp.org/sc?id=267) and to begin the incompatible with each other. initial steps to prepare for these changes. 10 mL syringes for medication administration via venous access devices not needed Some nurses erroneously believe that a 10 mL While smaller diameter syringes create syringe (or 10 mL diameter equivalent) is greater amounts of pressure than larger needed to administer IV medications via diameter syringes, the Infusion Nurses venous access devices (VAD). This has led to Society (INS) recommends flushing a VAD nurses emptying the contents of medications using a 10 mL syringe to assess patency. in smaller, prefilled 10 mL syringes into larger Thus, once patency is assured, medication syringes. This practice does not allow bedside administration in a smaller syringe is barcode scanning of the medication syringe acceptable. One of the largest and can lead to partial loss of a dose, manufacturers of IV access devices, Bard, contamination of the solution, inaccurate will be changing product labeling to clarify measurement of small doses, and unlabeled this issue. syringes. (21) To function at its full, safe potential, smart infusion pumps require entry of dose limit settings in their libraries for each high-alert IV medication infused by the pump. This can be a complex, time-consuming, and error-prone task. Individual hospital efforts also suggest wide variability in how the dose limits are expressed. (20) A nurse accidentally injected a hepatitis C positive patient with an empty insulin syringe before she realized she hadn’t filled it with insulin. She then used the same syringe and needle to withdraw insulin from a vial. That vial was later used January 30, 2014 Toolkit for smart pump dose limits The San Diego Patient Safety Council posted a toolkit (www.ismp.org/sc?id=255) to provide evidenced-based recommendations and best practices for establishing and managing highalert IV medication dose limit settings. The kit provides drug-specific recommendations for implementing dosing limits in your institution. Possible cross-contamination with insulin vials Using 3 mL insulin vials would expose fewer patients if the vial becomes contaminated. Where possible, preparation of patient-specific insulin doses by pharmacy would greatly reduce the risk of cross-contamination. ISMP MedicationSafetyAlert! QAA 2 October - December 2013 ISMP Problem No. QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed to prepare doses for other patients, exposing more than 70 patients to hepatitis C. (21) A pharmacy found a box of RabAvert with the diluent vial but not the actual vaccine powder. This could have resulted in an ineffective vaccination if only the diluent was administered. The diluent and vaccine vials are both packaged in small vials with similar green labels and caps. A fatal outcome is almost certain in people infected with rabies virus who do not receive post-exposure vaccination. (21) A pharmacy inadvertently dispensed an entire 1 mL vial of tuberculin 5 units/0.1 mL (10 doses) as a single dose. Only on the front of the vial’s label does it state 5 units/0.1 mL. The vial must be turned to see the notation of 10 doses per vial. (23) A nurse needed to administer ciprofloxacin but could not find the drug in the smart pump’s library. Fortunately, she did not simply override the dose-checking function and administer the drug. Follow-up showed the pump belonged to another hospital and was brought into the facility during a patient transfer. The two pumps looked identical, with the only difference being the respective organizations’ name displayed on the very top of the screen. January 30, 2014 RABAVERT (rabies vaccine) diluent could be mistaken as vaccine We have contacted Novartis to request improvements to the packaging. Hospitals may want to consider purchasing IMOVAX, a rabies vaccine produced by another company, which is presented in a sealed tray that holds a prefilled diluent syringe for reconstitution of the vaccine. Tuberculin purified protein derivative (PPD) label confusion All PPD doses should be dispensed from the pharmacy in unit-dose, intradermal syringes containing the proper dose. Infusion pump with library from different hospital Establish procedures outlining how to handle infusion pumps (and other equipment) during patient transfer. Consider applying a large, auxiliary label with your hospital’s name on all pumps that are owned. If rental pumps are used, they should arrive at the hospital with a blank library. If not, have the library removed immediately by biomedical engineering. The hospital-specific library should be loaded prior to use. ISMP MedicationSafetyAlert! QAA 3 October - December 2013 ISMP No. Problem (23) The recommended dose of Spiriva is 2 inhalations of the powder contents of 1 capsule. Many order entry systems will default to a dose of 1 inhalation, and if this is changed to 2 inhalations, it may result in the patient receiving the contents of 2 capsules. If the dose is entered as 1 capsule, the patient may receive the product orally. (24) QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed Potential dose confusion with SPIRIVA (tiotropium) Make sure Spiriva dosing is expressed clearly in your order entry system to avoid administering the wrong dose or via the wrong route. Express the dose in a manner that lessens the risk of confusion (e.g., 1 capsule = 2 inhalations). Ensure the patient understands how to properly use the medication (and inhalation device). Caution with Demo-Dose products (demonstration only) from Pocket Nurse A demo product for EPINEPHrine injection If your education department, simulation emergency syringes (Pocket Nurse) was lab, or associated nursing school is using found in a code cart. The demo product these or other demo products, be sure they looks nearly identical to syringes are strictly limited to classroom use. (Abboject) from Hospira that contain the Instructors should account for each demo actual drug. The Demo-Dose product is product at the end of class to ensure they sold for use during simulation training. Had don’t inadvertently reach a patient care these remained in the cart, they could have area. been injected during a code or delayed emergency treatment. Also, the distilled water in these demo syringes is not sterile. (22) An independent double-check cannot be performed for selected high-alert medications while working alone. (24) As the US Food and Drug Administration Performing a cognitive check while working alone Perform a cognitive check (left brain, right brain) if working alone by holding the container in one hand while reading the label aloud, and then switching hands and reading it aloud again. AVANDIA (rosiglitazone) and COUMADIN (warfarin) mix-ups with handwritten orders January 30, 2014 Most physician practices and many hospitals ISMP MedicationSafetyAlert! QAA 4 October - December 2013 ISMP QuarterlyActionAgenda Organization Assessment Problem Recommendation (FDA) lifts some of the restrictions previously in place with Avandia, the drug’s use may rise. Practitioners may not remember that Avandia and Coumadin were one of the most commonly reported serious drug name mixups in the past with handwritten orders. that are now utilizing electronic prescribing systems will not see this error. However, if the use of Avandia is being contemplated in the future, the potential for drug name mix-ups with handwritten prescriptions should be considered and addressed. (22) Errors are possible because apothecary strengths such as “gr” (grains) are still used for certain drugs such as ferrous sulfate and PHENobarbital. Grains (gr) could be confused with grams (g). Apothecary strengths confused Consider purchasing products from manufacturers that do not use apothecary strengths on labels to minimize the risk of confusion. If this is not pos-sible, ensure that pharmacy labels for these products do not contain apothecary strengths. (24) An immunization-certified pharmacist was not aware that all influenza vaccine products had to be shaken before use. The vaccine cartons are labeled “shake well.” But the vial and syringe labels of influenza vaccine that we’ve looked at are not so labeled. (23) Mix-ups have been reported between Cardizem and Cardene. In one case, an emergency department nurse prepared and administered a Cardene infusion instead of a Cardizem infusion. In another case, a nurse programmed a smart pump to infuse Cardizem instead of Cardene. The patient received the correct drug at the wrong infusion rate. No. January 30, 2014 Action Required/ Assignment Date Completed Influenza vaccine–shake well! Ensure staff who vaccinate patients with the flu vaccine are aware that all influenza vaccines, including FLUBLOK (which is a solution, not a suspension), need to be shaken before use, whether packaged in a single dose or multiple dose vial, or a prefilled syringe. CARDIZEM (diltiazem) and CARDENE (niCARdipine) mix-ups Change the appearance of these drug names on computer screens, pump display screens, storage areas (including automated dispensing cabinets), pharmacy product labels, and medication administration records (MARs), using bold face, color, or tall man letters for the parts of the names that are different. Configure computer/pump screens to prevent look-alike drug name pairs from appearing consecutively. Be sure mnemonics for either of these drugs is not simply C-A-RD. ISMP MedicationSafetyAlert! QAA 5 October - December 2013 ISMP No. January 30, 2014 Problem QuarterlyActionAgenda Organization Assessment Recommendation ISMP MedicationSafetyAlert! Action Required/ Assignment Date Completed QAA 6