Procedure - UNM Health Sciences Center
advertisement

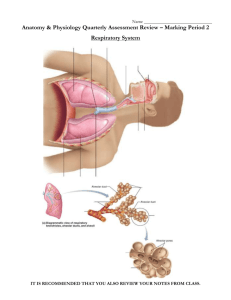
Applies To: All HSC Hospitals, CRTC, or UNMH Component(s): Responsible Department: Rapid Response Laboratory Title: Point of Care Testing Urinalysis Dipstick Test Patient Age Group: ( ) N/A (X ) All Ages ( ) Newborns Procedure ( ) Pediatric ( ) Adult DESCRIPTION/OVERVIEW 1. PURPOSE 1.1. Bayer Reagent Strips for Urinalysis are firm plastic strips to which are affixed several separate reagent areas. Test results may provide information regarding the status of carbohydrate metabolism, kidney and liver function, acid-base balance, urinary tract infection, urine concentration and carbohydrate metabolism. 2. PRINCIPLE 2.1. Glucose 2.1.1. This test is based on a double sequential enzyme reaction. One enzyme, glucose oxidase, catalyzes the formation of gluconic acid and hydrogen peroxide from the oxidation of glucose. A second enzyme, peroxidase, catalyzes the reaction of hydrogen peroxide with a potassium iodide chromogen to oxidize the chromogen to colors ranging from green to brown. 2.2. Bilirubin 2.2.1. This test is based on the coupling of bilirubin with diazotized dichloroaniline in a strongly acid medium. The color ranges through various shades of tan. 2.3. Ketone 2.3.1. This test is based on the development of colors ranging from buff-pink, for a negative reading, to purple when acetoacetic acid reacts with nitroprusside. 2.4. Specific Gravity 2.4.1. This test is based on the apparent pKa change of certain pretreated polyelectrolytes in relation to ionic concentration. In the presence of an indicator, colors range from deep blue-green in urine of low ionic concentration through green and yellow-green in urines of increasing ionic concentration. 2.5. Blood 2.5.1. This test is based on the peroxidase-like activity of hemoglobin, which catalyzes the reaction of diisopropylbenzene dihydroperoxide and 3,3’,5,5’tetramethylbenzidine. The resulting color ranges from orange through green; very high levels of blood may cause the color development to continue to blue. 2.6. pH 2.6.1. The test is based on the double indicator principle that gives a broad range of colors covering the entire urinary pH range. Colors range from orange through yellow and green to blue. 2.7. Protein 2.7.1. This test is based on the protein-error-of-indicators principle. At a constant pH, the development of any green color is due to the presence of protein. Colors range from yellow for “negative” through yellow-green and green to green-blue for “positive” reactions. Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 1 of 8 2.8. Urobilinogen 2.8.1. This test is based on a modified Ehrlich reaction, in which a ρdiethylaminobenzaldehyde in conjunction with a color enhancer reacts with urobilinogen in a strongly acid medium to produce a pink-red color. 2.9. Nitrite 2.9.1. This test depends upon the conversion of nitrate (derived from the diet) to nitrite by the action of Gram negative bacteria in the urine. At the acid pH of the reagent area, nitrite in the urine reacts with ρ-arsanilic acid to form a diazonium compound. This diazonium compound in turn couples with 1,2,3,4-tetrahydrobenzo(h)quinolin3-ol to produce a pink color. 2.10. Leukocytes 2.10.1. Granulocytic leukocytes contain esterases that catalyze the hydrolysis of the derivatized pyrrole amino acid ester to liberate 3-hydroxy-5-phenyl pyrrole. This pyrrole then reacts with a diazonium salt to produce a purple color. REFERENCES Bayer HealthCare Urine Dipstick Package Insert, Elhart, IN, 46514; 2005. AREAS OF REPONSIBILITY 1. POCT Urine Dipstick testing can be done on any inpatient or outpatient at University Hospital on receipt of an order from a physician or nursing staff designate. 2. Staff who have satisfied initial and annual competency requirements may perform the testing. PROCEDURE 1. SUPPLIES 1.1. Bayer Multistix 10SG or Uristix 4 1.2. Commercially available Normal and Abnormal Quality Control Solutions 2. ORDERING INFORMATION 2.1. Multistix and Uristix are ordered through Pharmacy 2.2. The Point of Care department provides quality control solutions. 3. STORAGE 3.1. Multistix and Uristix are stored at room temperature in the original bottle. Transfer to any other container may cause reagent strips to deteriorate and become unreactive. 3.2. Strips are stable until the expiration date printed on the bottle. Do not use expired strips. 3.3. Do not store the bottle in direct sunlight. Do not remove the desiccant from the bottle. 3.4. Quality control material will expire per manufacturer’s guidelines. 4. QUALITY CONTROL 4.1. The Point of Care department will perform quality control on each new lot number or shipment before patient testing is performed on that lot/shipment. 4.1.1. Notify Bayer HealthCare if shipment fails external quality control. 4.2. Each bottle of urine dipsticks will be QC’d when the testing department opens it. 4.2.1. Document on the bottle of urine dipsticks that QC was performed and the date. Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 2 of 8 4.3. One randomly selected department is required by the Point of Care Testing office to perform weekly quality control testing on one of their opened urine dipstick bottles. But all units performing patient testing are asked to also participate in weekly QC testing and documentation. 4.4. If the quality controls do not perform properly, repeat once. If the quality controls still do not perform properly do not perform patient testing on this bottle of urine dipsticks. 4.5. Document all results on the appropriate quality control log including any repeats or corrective actions taken. 4.6. Procedure: 4.6.1. Dip all of the test pads of the strip into the normal quality control solution. 4.6.2. Immediately remove the strip, dragging the edge of the strip against the container rim to remove excess urine. 4.6.3. Start timing. 4.6.4. Tap the strip on its side onto an absorbent cloth to remove excess urine. 4.6.5. Compare each test pad to the corresponding row of color blocks on the bottle label. 4.6.6. Read each pad at the time shown on the label, starting with the shortest time. 4.6.7. Hold the strip close to the color blocks and match carefully. 4.6.8. Repeat the procedure with the abnormal quality control solution. 5. PATIENT MANAGEMENT 5.1. Patient Identification 5.1.1. Patient identification may be the patient’s name, date of birth, medical record number, financial number or assigned trauma alert name. 5.1.2. The University of New Mexico Health Sciences Center (UNMHSC) does not recognize patients’ social security numbers as a patient identifier. 5.2. Patient Preparation 5.2.1. The operator must describe to the patient the purpose and steps of the procedure before testing can begin. 6. SPECIMEN COLLECTION AND HANDLING 6.1. Standard precautions apply to all point of care tests. 6.2. Testing personnel should handle all patient samples as per the Bloodborne Pathogen Exposure Plan. 6.3. Collect freshly-voided urine in a clean, dry container and test as soon as possible. 6.4. The container should allow for complete dipping of all reagent strip areas. 6.5. If unable to test immediately, refrigerate the specimen and let it return to room temperature before testing. 7. SPECIMEN LABELING 7.1. Specimens should be tested immediately at the patient’s bedside and discarded. 7.2. If immediate, bedside testing is not possible the sample should be labeled with two unique identifiers. 7.3. Patient identification may be the patient’s name, date of birth, medical record number or assigned trauma alert name. 7.4. UNMHSC does not recognize patients’ social security numbers as a patient identifier. 8. PATIENT TESTING Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 3 of 8 8.1. Collect a fresh urine specimen in a clean, dry container. 8.2. Mix well just before testing. 8.3. Remove one strip from the bottle. 8.4. Replace the cap. 8.5. Dip all the test pads of the strip into the urine. 8.6. Immediately remove the strip, dragging the edge of the strip against the container rim to remove excess urine. 8.7. Start timing. 8.8. Tap the strip on its side onto an absorbent cloth to remove excess urine. 8.9. Compare each test pad to the corresponding row of color blocks on the bottle label. 8.10. Read each pad at the time shown on the label, starting with the shortest time. 8.11. Hold the strip close to the color blocks and match carefully. 8.12. Read the pads in good light. 8.13. Record the results in the patient’s permanent medical record. 9. REFERENCE RANGE 9.1. All POC testing done in the hospital has a normal reference range validated with those tests performed in the laboratory. See the table below. 10. CRITICAL VALUES 10.1. Urinalysis dipstick testing is a qualitative assay used for screening purposes only. 10.2. All patient results should be reported to the physician or clinical personnel taking care of the patient. 10.3. Any patient result that is positive for Glucose and/or Ketones is considered a critical value and should be immediately reported to the clinical personnel taking care of the patient. The documentation in the patient’s chart should include the date, time, operator identification, patient identification, patient result, clinical personnel notified and corrective action. 11. RESULT REPORTING 11.1. Patient results should be immediately recorded in the permanent medical record. 11.2. Results that appear to be inconsistent with patient therapy or condition should be viewed as questionable and the test should be repeated by the laboratory reference method. NORMAL RANGE: pH: 5.0 – 8.0 Specific Gravity: 1.003 – 1.035 A normal dipstick is negative for leukocytes, nitrite, protein, glucose, ketones, urobilinogen, bilirubin and blood. REPORTABLE/SENSITIVITY RANGE: Reportable Range Protein: Negative – 2000 or more mg/dL Blood: Negative – Large/+++ Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 4 of 8 Sensitivity Range 15 – 30 mg/dL albumin 0.015 – 0.062 mg/dL Leukocytes: urine Nitrite: Glucose: Ketone: acid pH: Specific Gravity: Bilirubin: Urobilinogen: Negative – Large/+++ 5 – 15 WBC/hpf in clinical Negative – Positive Negative – 2000 or more mg/dL Negative – Large/160 mg/dL 0.06 – 0.1 mg/dL nitrite ion 75 – 125 mg/dL glucose 5 – 10 mg/dL acetoacetic 5.0 – 8.5 1.000 – 1.030 Negative – Large/+++ Negative – 8 mg/dL 5.0 – 8.5 1.001 – 1.035 0.4 – 0.8 mg/dL bilirubin ≥ 0.2 mg/dL 12. INTERFERING SUBSTANCES AND LIMITATIONS 12.1. Protection of test strips against exposure to light, heat and ambient moisture is mandatory to guard against altered reagent reactivity. 12.2. Substances that cause abnormal urine color may affect the readability of test pads on urinalysis reagent strips. These substances include visible level of blood or bilirubin and drugs containing dyes, nitrofurantoin or riboflavin. 12.3. Levels of ascorbic acid normally found in urine do not interfere with these tests. 12.4. Test pads should not be read after two minutes; color changes that occur after this time are of no diagnostic value. 12.5. Discoloration or darkening of the test pads may indicate deterioration. If this is evident, or if test results are questionable or inconsistent with expected findings, the following steps are recommended: 12.5.1. Confirm that the product is within the expiration date shown on the label 12.5.2. Check performance against known negative and positive control materials 12.5.3. Retest with fresh product 12.5.4. If proper results are not obtained, contact point of care testing at 2-0980. 12.6. Contamination of the urine specimen with skin cleansers containing chlorhexidine may affect protein (and to a lesser extent specific gravity and bilirubin) test results. The user should determine whether the use of such skin cleansers is warranted. 12.7. Fresh urine should be used for optimal results with the tests for bilirubin and urobilinogen, as these compounds are very unstable when exposed to room temperature and light. 12.8. Protein 12.8.1. A visibly bloody urine may cause falsely elevated results. 12.9. Blood 12.9.1. Capoten (captopril) may reduce the sensitivity. 12.9.2. Certain oxidizing contaminants, such as hypochlorite, may produce false positive results. 12.9.3. Microbial peroxidase associated with urinary tract infection may cause a false positive reaction. 12.10. Leukocytes 12.10.1. Elevated glucose concentrations (≥3 g/dL) may cause decreased test results. 12.10.2. The presence of cephalexin (Keflex), cephalothin (Keflin), or high concentrations of oxalic acid may also cause decreased test results. Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 5 of 8 12.10.3. Tetracycline may cause decreased reactivity, and high levels of the drug may cause a false negative reaction. 12.10.4. Positive results may occasionally be due to contamination of the specimen by vaginal discharge. 12.11. Nitrite 12.11.1. Pink spots or pink edges should not be interpreted as a positive result. 12.11.2. A negative result does not rule out significant bacteriuria. 12.11.3. False negative results may occur with shortened bladder incubation of the urine, absence of dietary nitrate, or the presence of nonreductive pathological microbes. 12.12. Glucose 12.12.1. Ketone bodies reduce the sensitivity of the test; moderately high ketone levels (40 mg/dL) may cause false negatives for specimens containing small amounts of glucose (75 – 125 mg/dL) but the combination of such ketone levels and low glucose levels is metabolically improbable in screening. 12.13. Ketone 12.13.1. False trace results may occur with highly pigmented urine specimens or those containing large amounts of levodopa metabolites. 12.13.2. Compounds such as mesna (2-mercaptoethane sulfonic acid) that contain sulfhydryl groups may cause false positive results or an atypical color reaction. 12.14. pH 12.14.1. Bacterial growth by certain organisms in a specimen may cause a marked alkaline shift (pH >8.0), usually because of urea conversion to ammonia. 12.15. Specific Gravity 12.15.1. The Bayer SG test is dependent on ions in urine and results may differ from those obtained with other specific gravity methods when certain nonionic urine constituents, such as glucose, are present. 12.15.2. Highly buffered alkaline urines may cause low readings, while the presence of moderate quantities of protein (100 – 750 mg/dL) may cause elevated readings. 12.16. Bilirubin 12.16.1. Indican (indoxyl sulfate) can produce a yellow-orange to red color response that may interfere with the interpretation of a negative or positive reading. Metabolites of Lodine (etodolac) may cause false positive or atypical results. 12.16.2. Atypical colors (colors that are unlike the negative or positive color blocks shown on the color chart) may indicate that bilirubin-derived bile pigments are present in the urine sample and may be masking the bilirubin reaction. These colors may indicate bile pigment abnormalities and the urine specimen should be tested further. 12.17. Urobilinogen 12.17.1. The test pad may react with interfering substances known to react with Ehrlich’s reagent, such as ρ-aminosalicylic acid and sulfonamides. 12.17.2. Atypical color reactions may be obtained in the presence of high concentrations of ρ-aminobenzoic acid. 12.17.3. False negative results may be obtained if formalin is present. 12.17.4. Strip reactivity increases with temperature; the optimum temperature is 22° 26°C. The test is not a reliable method for the detection of porphobilinogen. CLIA Classification Waived Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 6 of 8 Use If definitive, how will results be used? Screen Provide information on the kidney, liver function, acid base balance, and urinary tract infection. DEFINITIONS None SUMMARY OF CHANGES Replaces POCT Urine Dipstick Testing, revision 5/15/06 KEY WORDS Dipstick, urine dipstick, multistix Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 7 of 8 RESOURCES/TRAINING Point of Care Testing, 2-0980 Resource/Dept Internet/Link DOCUMENT APPROVAL & TRACKING Owner Item Contact Point of Care Testing Coordinator Date Consultant(s) Tricore Reference Laboratories Committee(s) Point of Care Testing Advisory Committee [Y or N/A] Nursing Director Medical Director Human Resources Finance Legal Official Approver [Name], Chief Nursing Officer Glynnis Ingall, MD, PhD; Rapid Response Laboratory [Name], HR Administrator, [UNMH or UNM] [Name, Title], [UNMH or HSC] [Name, Title], [UNMH or HSC] [Name, Title, Area] [Y or N/A] [Y or N/A] [Y or N/A] [Y or N/A] [Y or N/A] Y [Day/Mo/Year] Official Signature Approver (Optional) Signature [Day/Mo/Year] Effective Date Origination Date Issue Date [Day/Mo/Year] [Month/Year] [Day/Mo/Year] Clinical Operations P&P Coordinator ATTACHMENTS None. Title: Point of Care Testing: Urinalysis Dipstick Test Owner: Point of Care Testing Coordinator Effective Date: Page 8 of 8 Approval