EXPERIMENT 11: OXIDATION OF ALCOHOLS
advertisement

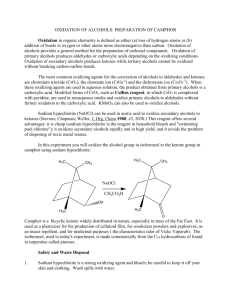
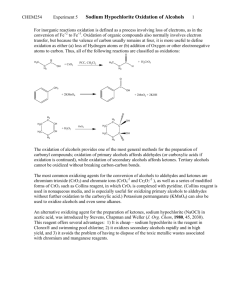
Lab 9: SODIUM HYPOCHLORITE OXIDATION: PREPARATION OF CAMPHOR From: http://www.wou.edu/las/physci/ch335/preparation%20of%20camphor.doc Oxidation has a somewhat different meaning in organic chemistry than you have been used to in inorganic, where oxidation is defined as a process involving loss of electrons, as in the conversion of Fe+2 to Fe+3. Oxidation of organic compounds also normally involves electron transfer, but because the valence of carbon usually remains at four, it is more useful to define oxidation as either (a) loss of hydrogen atoms or (b) addition of oxygen or other electronegative atoms. Thus, all of the following reactions are classified as oxidations: H CrO3 O OH OH KMnO4 C CH 3 O OH H2O2 OsO4 OH Oxidations of alcohols provides one of the most general methods for the preparation of carbonyl compounds; oxidation of primary alcohols affords aldehydes (or carboxylic acids, if oxidation is continued), while oxidation of secondary alcohols affords ketones. Tertiary alcohols cannot be oxidized without breaking carbon-carbon bonds. H R Aldehyde Primary alcohol R' R O R OH OH R O Carboxylic Acid R' OH R O The most common oxidizing agents for the conversion of alcohols to aldehydes and ketones are chromium trioxide (CrO3) and its relatives, chromate and dichromate ions (CrO4-2 and Cr2O7-2), as well as a series of modified forms of CrO3, such as Collins reagent, in which CrO3 is complexed with pyridine (Collins reagent is used in nonaqueous media, and is especially useful for oxidizing primary alcohols to aldehydes without overoxidation to the carboxylic acid). KMnO4 can also be used to oxidize alcohols. 1 An alternative oxidizing agent for the preparation of ketones, sodium hypochlorite (NaOCl) in acetic acid, was introduced by Stevens, Chapman, and Weller in 1980 (J. Org. Chem. 45, 2030). This reagent offers several advantages: it is cheap (sodium hypochlorite is the reagent in Clorox and "swimming pool chlorine"); it oxidizes secondary alcohols rapidly and in high yield; and it avoids the problem of disposing of toxic wastes associated with chromium and manganese reagents. The example chosen to illustrate the oxidation of alcohols is the sodium hypochlorite oxidation of isoborneol to camphor: H3C H3C CH 3 CH 3 NaOCl CH3CO2H OH H3C H3C O Camphor is a bridged bicyclic ketone widely distributed in nature, especially in trees of the Far East. It is used as a plasticizer for the production of celluloid film, for smokeless powders and explosives, as an insect repellent, and for medicinal purposes (you will recognize the characteristics odor of Vicks). Borneol, one of the stereoisomeric related alcohols, is found in certain trees of Borneo, and isoborneol, used in today's experiment, is made commercially from pinenes (the C10 hydrocarbons of turpentine). Safety and Waste Disposal 1. Sodium hypochlorite (Clorox) is a strong oxidizing agent and bleach; be careful to keep it off your skin and clothing. Wash spills with water. 2. Excess Clorox, as well as the filtrate from the collection of camphor, can be poured down the drain if diluted well with water. 3. If you distill the methylene chloride at the end of the experiment, put the distillate in the bottle labeled "RECOVERED METHYLENE CHLORIDE" 2 EXPERIMENTAL PROCEDURE Dissolve 5 g of isoborneol in 15 mL of glacial acetic acid in a 125-mL Erlenmeyer flask. Add 50 mL of Clorox by the milliliter over 5 minutes, cooling the flask as necessary to keep the internal temperature in the range 15-25ºC. A white precipitate will separate out at this point. Allow the mixture to stand at room temperature for 30 mins with swirling (every 3 mins). A positive KIstarch test should be obtained at this point (White KI-starch paper will turn blue-violet for positive test). Add saturated NaHSO3 (Sodium bisulfite: Quenches excess oxidizing agent) solution carefully until the yellow color of the reaction mixture disappears and the KI-starch test is negative (White paper stays white). Pour the mixture over 100 mL of brine (Saturated NaCl) and ice[take 100 ml brine and add a fistful of ice], collect the solid by filtration on a Buchner funnel, and wash it with saturated NaHCO3 (Sodium bicarbonate) solution until foaming is no longer evident. Air-dry your solid for 15 minutes (use high vacuum) by using the Buchner filtration system. Weigh the dry product so that you can calculate the yield. Wait until the product dries above; you will need to get all the material needed for this step! Once the product dries, you will need to prepare the 2,4 Dinitrophenyl hydrazone derivative of camphor: Dissolve about 0.2 g of your camphor in 5 mL of 95% ethanol, add 10 mL of the 2,4dinitrophenylhydrazine reagent solution, heat to boiling on a hot plate for 3 minutes (The solution will turn deep yellow or yellow-orange at this point. Don’t worry if some of you don’t observe this. You will see crystals upon cooling the flask on ice!), and allow the mixture to cool (1-2 min). Scratch the bottom of the flask with a clean spatula and keep the flask on ice. Crystallization (yellowish crystals) usually occurs within a few minutes. Filter the yellow precipitate by suction filtration, wash with 2-3 mL of chilled water, and allow drying to occur (high vacuum for 5 min). Determine the melting point (reported mp 164 oC) of your 2,4-DNPH-derivative of camphor from above. Calculate the percent yield of your product (camphor). Post-lab Questions: (Answer these in your notebook). 1. Write a balanced equation for the oxidation of isoborneol by NaOCl. 2. What do you actually observe in a "positive KI-starch test"? What does it mean when it is mentioned at the end of the first paragraph in the Experimental Procedure that the KI-Starch test is positive? 3 3. What is the purpose of adding NaHSO3 at the end of the oxidation? Write a balanced equation for the reaction it undergoes. Notes. 1. Commercial Clorox contains about 6.5% NaOCl. 2. Preparation of 2,4-dinitrophenylhydrazine reagent: Add 50 mL of concentrated sulfuric acid to 10 g of 2,4-dinitrophenylhydrazine in a 500-mL Erlenmeyer flask and mix well. Add 75 mL of water dropwise (CAREFUL! HEAT EVOLUTION), with stirring or swirling, until solution is complete. To this warm solution add 250 mL of 95% ethanol. This scale provides enough reagents for 35 students. The solution should be prepared fresh each day. 3. For alternative oxidations of secondary alcohols with Clorox, see (a) Oxidation of cyclohexanol: N.M. Zuczek and P.S. Furth, in J. Chem Educ. 58, 824 (1981). (b) Oxidation of cyclic and acyclic secondary alcohols: R.A. Perkins and F. Chau, in J. Chem. Edu. 59, 981 (1982). (c) Oxidation of 9-fluorenol: C.S. Jones and K. Albizati, in J. Chem. Educ. 71, A271 (1994). 4