הודעה על החמרה ( מידע בטיחות) בעלון לצרכן
advertisement

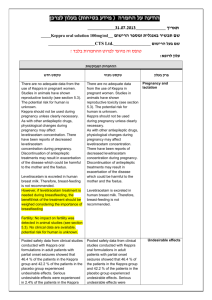
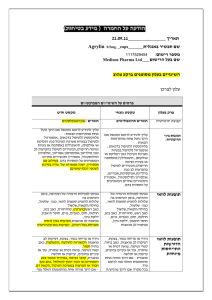
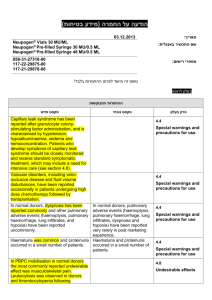
.08..80.50 :תאריך Iclusig 15 and Iclusig 45 שם תכשיר באנגלית ומספר הרישום 511-11-14241-05 ,511-11-14241-00 : מ"ג51 איקלוסיג 511-14-14244-05 ,511-14-14244-00 : מ"ג41 איקלוסיג פתח,50 רח' השילוח, אריאד פרמצבטיקה (ישראל) בע"מ. איי. פי. אי: שם בעל הרישום תקווה ! טופס זה מיועד לפרוט ההחמרות בלבד רופא בעלון ללרופא בטיחות) בעלון )מידע בטיחות החמרה (( מידע על החמרה הודעה על הודעה ההחמרות המבוקשות טקסט חדש טקסט נוכחי Vascular occlusion Arterial and venous thrombosis and occlusions, including fatal myocardial infarction, stroke, retinal vascular occlusions associated in some cases with permanent visual impairment or vision loss, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures have occurred in Iclusig-treated patients. Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. Vascular occlusion adverse events were more frequent with increasing age and in patients with prior history of ischaemia, hypertension, diabetes, or hyperlipidaemia. Vascular occlusion Arterial and venous thrombosis and occlusions, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures have occurred in Iclusig-treated patients. Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. Vascular occlusion adverse events were more frequent with increasing age and in patients with prior history of ischaemia, hypertension, diabetes, or hyperlipidaemia. Monitoring for evidence of thromboembolism and vascular occlusion should be performed and if decreased vision or blurred vision occurs, an ophthalmic examination (including fundoscopy) should be performed. Iclusig should be interrupted immediately in case of vascular occlusion. A benefit -risk consideration should guide a decision to restart Iclusig therapy (see sections 4.2 and 4.8). Monitoring for evidence of thromboembolism and vascular occlusion should be performed an. Iclusig should be interrupted immediately in case of vascular occlusion. A benefit -risk consideration should guide a decision to restart Iclusig therapy (see sections 4.2 and 4.8). Hypertension Hypertension may contribute to risk of arterial thrombotic events. During Iclusig treatment, blood pressure should be monitored and managed at each clinic visit and hypertension should be treated to normal. Iclusig treatment should be temporarily interrupted if hypertension is not medically controlled (see section 4.2). Hypertension may contribute to risk of arterial thrombotic events. During Iclusig treatment, blood pressure should be monitored and managed at each clinic visit and hypertension should be treated to normal. Iclusig treatment should be temporarily interrupted if hypertension is not medically controlled (see section 4.2). פרק בעלון 4.4 Special warnings and precautions for use Treatment-emergent hypertension (including hypertensive crisis) occurred in Iclusig-treated patients. Patients may require urgent clinical intervention for hypertension associated with confusion, headache, chest pain, or shortness of breath. Treatment-emergent hypertension occurred in Iclusig-treated patients. Patients may require urgent clinical intervention for hypertension associated with confusion, headache, chest pain, or shortness of breath. Hepatotoxicity Liver function abnormality Iclusig may result in elevation in ALT, AST, bilirubin, and alkaline phosphatase. Hepatic failure (including fatal outcome) has been observed. Liver function tests should be performed prior to treatment initiation and monitored periodically, as clinically indicated. Liver function abnormality Iclusig may result in elevation in ALT, AST, bilirubin, and alkaline phosphatase. Liver function tests should be performed prior to treatment initiation and monitored periodically, as clinically indicated. מצ"ב טבלה מצ"ב טבלה Undesirable effects Current: Table 3 Adverse reactions observed in CML and Ph+ ALL patients – frequency reported by incidence of treatment emergent events System organ class Infections and infestations Frequency Very common Common Very common Blood and lymphatic system disorders Common Very common Metabolism and nutrition disorders Common Psychiatric disorders Uncommon Very common Very common Nervous system disorders Common Uncommon Adverse reactions upper respiratory tract infection pneumonia, sepsis, folliculitis anaemia, platelet count decreased, neutrophil count decreased pancytopenia, febrile neutropenia, white blood cell count decreased decreased appetite dehydration, fluid retention, hypocalcaemia, hyperglycaemia, hyperuricaemia, hypophosphataemia, hypertriglyceridaemia, hypokalaemia, weight decreased tumour lysis syndrome insomnia headache, dizziness cerebrovascular accident, cerebral infarction, neuropathy peripheral, lethargy, migraine, hyperaesthesia, hypoaesthesia, paraesthesia, transient ischaemic attack cerebral artery stenosis System organ class Frequency Common Eye disorders Uncommon Common Cardiac disorders Uncommon Very common Common Vascular Disorders Uncommon Very common Respiratory, thoracic and mediastinal disorders Common Very common Gastrointestinal disorders Common Uncommon Very common Hepatobiliary disorders Common Uncommon Very common Skin and subcutaneous tissue disorders Musculoskeletal and connective tissue disorders Reproductive system and breast disorders General disorders and administrative site conditions Common Very common Common Common Very common Common Adverse reactions vision blurred, dry eye, periorbital oedema, eyelid oedema retinal vein thrombosis, retinal vein occlusion, retinal artery occlusion, visual impairment cardiac failure, myocardial infarction, cardiac failure congestive, coronary artery disease, angina pectoris, pericardial effusion, atrial fibrillation, ejection fraction decreased myocardial ischemia, acute coronary syndrome, cardiac discomfort, ischemic cardiomyopathy, arteriospasm coronary, left ventricular dysfunction, atrial flutter hypertension peripheral arterial occlusive disease, peripheral ischaemia, peripheral artery stenosis, intermittent claudication, deep vein thrombosis, hot flush, flushing poor peripheral circulation, splenic infarction, embolism venous, venous thrombosis dyspnoea, cough pulmonary embolism, pleural effusion, epistaxis, dysphonia, pulmonary hypertension abdominal pain, diarrhoea, vomiting, constipation, nausea, lipase increased pancreatitis, blood amylase increased, gastrooesophageal reflux disease, stomatitis, dyspepsia, abdominal distension, abdominal discomfort, dry mouth gastric haemorrhage alanine aminotransferase increased, aspartate aminotransferase increased blood bilirubin increased, blood alkaline phosphatase increased, gammaglutamyltransferase increased hepatotoxicity, jaundice rash, dry skin rash pruritic, exfoliative rash, erythema, alopecia, pruritis, skin exfoliation, night sweats, hyperhidrosis, petechia, ecchymosis, pain of skin, dermatitis exfoliative bone pain, arthralgia, myalgia, pain in extremity, back pain, muscle spasms musculoskeletal pain, neck pain, musculoskeletal chest pain erectile dysfunction fatigue, asthenia, oedema peripheral, pyrexia, pain chills, influenza like illness, non-cardiac chest pain, mass, face oedema Updated: Table 3 Adverse reactions observed in CML and Ph+ ALL patients – frequency reported by incidence of treatment emergent events System organ class Infections and infestations Frequency Very common Common Very common Blood and lymphatic system disorders Common Very common Metabolism and nutrition disorders Common Psychiatric disorders Uncommon Very common Very common Nervous system disorders Common Uncommon Common Eye disorders Uncommon Common Cardiac disorders Uncommon Very common Common Vascular Disorders Uncommon Very common Respiratory, thoracic and mediastinal disorders Common Adverse reactions upper respiratory tract infection pneumonia, sepsis, folliculitis anaemia, platelet count decreased, neutrophil count decreased pancytopenia, febrile neutropenia, white blood cell count decreased decreased appetite dehydration, fluid retention, hypocalcaemia, hyperglycaemia, hyperuricaemia, hypophosphataemia, hypertriglyceridaemia, hypokalaemia, weight decreased tumour lysis syndrome insomnia headache, dizziness cerebrovascular accident, cerebral infarction, neuropathy peripheral, lethargy, migraine, hyperaesthesia, hypoaesthesia, paraesthesia, transient ischaemic attack cerebral artery stenosis vision blurred, dry eye, periorbital oedema, eyelid oedema retinal vein thrombosis, retinal vein occlusion, retinal artery occlusion, visual impairment cardiac failure, myocardial infarction, cardiac failure congestive, coronary artery disease, angina pectoris, pericardial effusion, atrial fibrillation, ejection fraction decreased myocardial ischemia, acute coronary syndrome, cardiac discomfort, ischemic cardiomyopathy, arteriospasm coronary, left ventricular dysfunction, atrial flutter hypertension peripheral arterial occlusive disease, peripheral ischaemia, peripheral artery stenosis, intermittent claudication, deep vein thrombosis, hot flush, flushing poor peripheral circulation, splenic infarction, embolism venous, venous thrombosis, hypertensive crisis dyspnoea, cough pulmonary embolism, pleural effusion, epistaxis, dysphonia, pulmonary hypertension System organ class Frequency Very common Gastrointestinal disorders Common Uncommon Very common Hepatobiliary disorders Common Uncommon Very common Skin and subcutaneous tissue disorders Musculoskeletal and connective tissue disorders Reproductive system and breast disorders General disorders and administrative site conditions Common Very common Common Common Very common Common Adverse reactions abdominal pain, diarrhoea, vomiting, constipation, nausea, lipase increased pancreatitis, blood amylase increased, gastrooesophageal reflux disease, stomatitis, dyspepsia, abdominal distension, abdominal discomfort, dry mouth gastric haemorrhage alanine aminotransferase increased, aspartate aminotransferase increased blood bilirubin increased, blood alkaline phosphatase increased, gammaglutamyltransferase increased hepatotoxicity, hepatic failure, jaundice rash, dry skin rash pruritic, exfoliative rash, erythema, alopecia, pruritis, skin exfoliation, night sweats, hyperhidrosis, petechia, ecchymosis, pain of skin, dermatitis exfoliative bone pain, arthralgia, myalgia, pain in extremity, back pain, muscle spasms musculoskeletal pain, neck pain, musculoskeletal chest pain erectile dysfunction fatigue, asthenia, oedema peripheral, pyrexia, pain chills, influenza like illness, non-cardiac chest pain, mass, face oedema לצרכן בעלון לצרכן בטיחות) בעלון )מידע בטיחות החמרה (( מידע על החמרה על ההחרמות המבוקשות טקסט חדש :תופעות לוואי לא שכיחות טקסט נוכחי פרק בעלון : תופעות לוואי תופעות לוואי לא שכיחות.4 הפרעות מטבוליות הנגרמות מתוצרי פירוק של תאי סרטן הפרעות מטבוליות הנגרמות מתוצרי פירוק של תאי סרטן , חסימת כלי דם בעיניים, היצרות של העורקים במוח, חסימת כלי דם מתים, היצרות של העורקים במוח,מתים כאב, בעיות בכלי דם בשריר הלב, בעיות לב, בעיות בכלי דם הפרעות בראייה, בעיות לב, הפרעות בראייה,בעיניים בעיות בתפקוד החדר השמאלי של, בעיות בתפקוד בצד שמאל של החזה, כאב בצד שמאל של החזה,בשריר הלב עליה פתאומית, הפרעות בזרימת הדם, הצרות כלי דם,הלב הפרעות בזרימת, הצרות כלי דם,החדר השמאלי של הלב דימום מהקיבה, בעיות בזרימת הדם בטחול,בלחץ דם דימום מהקיבה, בעיות בזרימת הדם בטחול,הדם צהבת, נזק כבדי,) דם בקיא, צהבת (סימפטומים – כאב בטן, נזק כבדי,) דם בקיא,(סימפטומים – כאב בטן )(מתבטאת בהצהבת העור והעיניים )(מתבטאת בהצהבת העור והעיניים . שבו מסומנות ההחמרות המבוקשות על רקע צהוב,מצ"ב העלון יש לסמן רק תוכן מהותי ולא שינויים במיקום.שינויים שאינם בגדר החמרות סומנו (בעלון) בצבע ירוק .הטקסט