הודעה על החמרה ( מידע בטיחות) בעלון לצרכן
advertisement

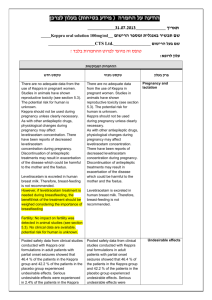
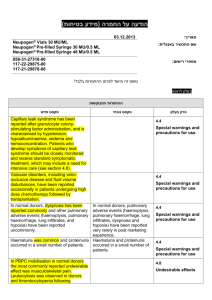
הודעה על החמרה ( מידע בטיחות) בעלון לרופא )05.2013 (מעודכן 21/07/2013 :תאריך Removab concentrate for solution for infusion – 146 57 33255 :שם תכשיר באנגלית ומספר הרישום Neopharm Scientific Ltd. :שם בעל הרישום ! טופס זה מיועד לפרוט ההחמרות בלבד ההחמרות המבוקשות טקסט חדש טקסט נוכחי Women of childbearing potential Removab is not recommended in women of childbearing potential not using contraception. Women of childbearing potential Removab is not recommended in women of childbearing potential not using contraception. Pregnancy There are no or limited amount of data from the use of catumaxomab in pregnant women. Animal studies are insufficient with respect to reproductive toxicity (see section 5.3). Removab is not recommended during pregnancy and in women of childbearing potential not using contraception. Pregnancy There are no or limited amount of data from the use of catumaxomab in pregnant women. Animal studies are insufficient with respect to reproductive toxicity (see section 5.3). Removab is not recommended during pregnancy. פרק בעלון 4.6 Fertility, pregnancy and lactation b) Tabulated list of adverse reactions The adverse reactions listed below are derived from an integrated safety analysis including 1112 clinical studies. 517728 patients received Removab in intraperitoneally, 293 patients as 6 hour - and 224435 patients as 3 hour infusions. b) Tabulated list of adverse reactions The adverse reactions listed below are derived from an integrated safety analysis including 11 clinical studies. 517 patients received Removab in intraperitoneally, 293 patients as 6 hour and 224 patients as 3 hour infusions. Infections and infestations: Common: Infection, urinary tract infection Blood and lymphatic system disorders Common: Anaemia*, lymphopenia, leukocytosis, neutrophilia, thrombocytosis. Uncommon: Thrombocytopenia*, coagulopathy*. Immune system disorders Common: Cytokine release syndrome*, hypersensitivity*. Metabolism and nutrition disorders Common: Decreased appetite* / anorexia, dehydration*, hypokalaemia, hypoalbuminaemia, hyponatraemia*, hypocalcaemia*, hypoproteinaemia, hyperglycaemia, hypoalbuminaemia. Vascular disorders Common: Hypotension*, hypertension*, flushing, hot flush. Respiratory, thoracic and mediastinal disorders Common: Dyspnoea*, pleural effusion*, hypoxia*, cough. Uncommon: Pulmonary embolism*, hypoxia*. Gastrointestinal disorders Common: Constipation*, dyspepsia, abdominal distension, Infections and infestations: Common: Infection, urinary tract infection Blood and lymphatic system disorders Common: Anaemia, lymphopenia, leukocytosis, neutrophilia, thrombocytosis. Uncommon: Thrombocytopenia* Immune system disorders Common: Cytokine release syndrome*, hypersensitivity. Metabolism and nutrition disorders Common: Decreased appetite* / anorexia, dehydration*, hypokalaemia, hyponatraemia, hypocalcaemia, hypoproteinaemia, hyperglycaemia, hypoalbuminaemia. Vascular disorders Common: Hypotension*, hypertension*, flushing, hot flush. Respiratory, thoracic and mediastinal disorders Common: Dyspnoea*, pleural effusion*, hypoxia*, cough. Uncommon: Pulmonary embolism* Gastrointestinal disorders Common: Constipation*, abdominal distension, dyspepsia, 4.8 Undesirable effects dyspepsia,sub-ileus*, flatulence, ileus*, sub-ileus*, gastric disorder, ileus*, gastroesophageal reflux disease, dry mouth. Skin and subcutaneous tissue disorders Common: Rash*, erythaema*, pruritus, hyperhidrosis, dermatitis allergic*.pruritus Uncommon: Skin reaction*, dermatitis allergic*. Renal and urinary disorders Common: Proteinuria, haematuria, leukocyturia General disorders and administration site conditions Very Common: Pyrexia*, fatigue*, chills*, pain. Common: Pain, asthenia*, Systemic inflammatory response syndrome*, asthenia, oedema incl. oedema peripheral,*, general physical health deterioration*, chest pain, influenza-like illness, malaise,*, catheter site erythema. Uncommon: Extravasation*, application site inflammation*, general physical health deterioration*. flatulence, ileus*, sub-ileus*, gastric disorder, gastroesophageal reflux disease. Skin and subcutaneous tissue disorders Common: Rash*, erythaema*, pruritus, hyperhidrosis, dermatitis allergic*. Uncommon: Skin reaction*. Renal and urinary disorders Common: Proteinuria, haematuria, leukocyturia. General disorders and administration site conditions Very Common: Pyrexia*, fatigue*, chills*, pain. Common: Systemic inflammatory response syndrome*, asthenia, oedema incl. oedema peripheral, chest pain, influenza-like illness, malaise, catheter site erythema. Uncommon: Extravasation*, application site inflammation*, general physical health deterioration*. Cytokine release related symptoms with higher intensities: In 4.45.1% of patients pyrexia reached an intensity of CTCAE grade 3 as it was the case with cytokine release syndrome (1.20%), chills (1.0.8%), nausea (3.74%), vomiting (5.64.4%), dyspnoea (1.46%) and hypo-/hypertension (2.1.9% / 1.2% / 0.8%). In one patient (0.2%) dsyspnoea1%) dyspnoea and in 3 patients (0.64%) hypotension was reported in CTCAE grade 4 intensity. Symptoms of pain and pyrexia can be ameliorated or avoided by premedication (see sections 4.2 and 4.4). Cytokine release related symptoms with higher intensities: In 4.4% of patients pyrexia reached an intensity of CTCAE grade 3 as it was the case with cytokine release syndrome (1.2%), chills (1.0%), nausea (3.7%), vomiting (5.6%), dyspnoea (1.4%) and hypo-/hypertension (1.9% / 1.2%). In one patient (0.2%) dsyspnoea) and in 3 patients (0.6%) hypotension was reported in CTCAE grade 4 intensity. Symptoms of pain and pyrexia can be ameliorated or avoided by pre-medication (see sections 4.2 and 4.4). Systemic Inflammatory Response Syndrome (SIRS): In 4.63.8 % of the patients symptoms of SIRS were observed within 24 hours after Removab infusion. In three patients (0. 64 %) an Systemic Inflammatory Response Syndrome (SIRS): In 4.6% of the patients symptoms of SIRS were observed within 24 hours after Removab infusion. In three patients (0. 6 %) an intensity of CTCAE grade 4 was observed. These reactions resolved under symptomatic treatment. intensity of CTCAE grade 4 was observed. These reactions resolved under symptomatic treatment. Abdominal pain: In 48.443.7 % of patients abdominal pain was reported as an adverse reaction reaching grade 3 in 9.98.2 % of patients, but it resolved under symptomatic treatment. Abdominal pain: In 48.4. % of patients abdominal pain was reported as an adverse reaction reaching grade 3 in 9.9 % of patients, but it resolved under symptomatic treatment. Hepatic enzymes: Transient increase in hepatic enzymes was commonly observed after the administration of Removab. In general, the changes in laboratory parameters were not clinically relevant and mostly returned to baseline after end of treatment, Only in case of clinically relevant or persisting increase further diagnostics or therapy should be considered.