MICROSCOPY
advertisement

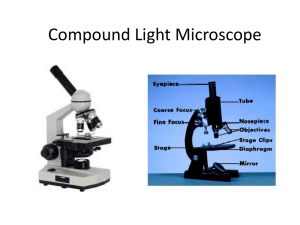
MICROSCOPY basic requirement-extra requirement THE LIGHT Light: electromagnetic radiation of a wavelength that is visible to the human eye (about 400– 700 nm). Light can exhibit properties of both waves and particles (photons). This property is referred to as wave–particle duality. Monochromatic light: light ray possessing one single wavelength. Complex light: mixture of light rays with more different wavelengths. Characteristic parameters of light waves: Wavelength: the distance between repeating units of a propagating wave of a given frequency. (λ, expressed in nm). Frequency: The number of oscillations within a minute. Amplitude: distance from the center y position to the peak MAGNIFICATION Total visual magnification of the microscope is derived by multiplying the magnification values of the objective and the eyepiece. (Ocular: 5-30 x magnification, Objective: 4-100 x magnification, Maximum magnification: 3000X). Can we apply the maximal magnification? No, because over 1000x- magnification we do not see more details, the points of the image just get bigger. Therefore the resolution is important, not the magnification. THE RESOLUTION Resolution: the shortest distance between two points on a specimen that can still be distinguished by the observer or camera system as separate entities. The resolution of our optical equipment is better as closer points can be seen as separate ones. he resolution of a good light microscope is ~0.25 m The resolution of the microscope, () : = /2n·sinα. The letter n is the refraction index of the media between the cover slip and the objective (air n=1, distilled water n=1.33, cedar oil n=1.51). α labels the angle closed by the main optical axis and the outermost light beam (half angle of the objective) POSSIBILITIES OF RESOLUTION IMPROVEMENT (i) Reduction of the numerator, i.e. application of light beam with a shorter wavelength. With UV light the resolution can be reduced to 0.1 μm, but special quartz lenses and UV-light detector are needed, therefore the light microscope with UV light source is only a theoretical possibility. (ii) To increase the value of the numeric aperture (A = n·sinα). Increasing n (refraction index) : The resolution of the microscope can be enhanced by dropping a solution with higher refractive index between the front lens and the coverslip. Those lenses (mainly objectives with 100x magnification) are named as immersion objectives. The immersion liquid mentioned above is cedar oil, thus these lenses are objectives with oil immersion (they are labeled with HI). For the WI labeled objectives distilled water is the liquid which should be used. Increasing α: The half angle of a lens can be increased only until 72°, since at larger angle than this the light beams became totally reflected. ADVANCED MICROSCOPY PHASE CONTRAST MICROSCOPY A large spectrum of living biological specimens are virtually transparent when observed in the optical microscope under brightfield illumination. Phase contrast microscopy provides an excellent method of improving contrast in unstained biological specimens without significant loss in resolution, and is widely utilized to examine dynamic events in living cells. Fritz Zernike received a Nobel prize in 1953 for his discovery of phase contrast. In a phase-contrast microscope, the annular rings in the objective lens and the condenser separate the light. The light that passes through the central part of the light path is recombined with the light that travels around the periphery of the specimen. The interference produced by these two paths produces images in which the dense structures appear darker than the background. FLUORESCENCE MICROSCOPY Fluorescence: The process by which a suitable atom or molecule, which is transiently excited by absorption of external radiation at the proper energy level (usually ultraviolet or visible light), releases the absorbed energy as a photon having a wavelength longer than the absorbed energy. The fluorescence excitation and emission processes usually occur in less than a nanosecond. Fluorescence Microscopy is the most rapidly expanding microscopy technique employed today, both in the medical and biological sciences. When coupled to the optical microscope, fluorescence enables investigators to study a wide spectrum of phenomena in cellular biology. Foremost is the analysis of intracellular distribution of specific macromolecules in subcellular assemblies, such as the nucleus, membranes, cytoskeletal filaments, mitochondria, Golgi apparatus, and endoplasmic reticulum. In addition to steady state observations of cellular anatomy, fluorescence is also useful to probe intracellular dynamics and the interactions between various macromolecules, including diffusion, binding constants, enzymatic reaction rates, and a variety of reaction mechanisms, in time-resolved measurements. For example, fluorescent probes have been employed to monitor intracellular pH and the localized concentration of important ions. Microscopes with an inverted-style frame are designed primarily for tissue culture applications and are capable of producing fluorescence illumination through an episcopic and optical pathway. Epi-illuminators usually consist of a mercury or xenon lamphouse (or laser system) stationed in a port at the rear of the microscope frame. Fluorescence illumination from the arc lamp passes through a collector lens and into a cube that contains a set of interference filters, including a dichroic mirror, barrier filter, and excitation filter. Light reflected from the dichroic mirror is restricted in wavelength by the excitation filter and enters the objective (now acting as a condenser) to bathe the specimen with a cone of illumination whose size and shape is determined by the objective numerical aperture. Secondary fluorescence, emitted by the specimen, returns through the objective, dichroic mirror and barrier filter before being routed through the microscope optical train. The microscope presented above contains a trinocular observation tube that is equipped with a port and extension tube for mounting a traditional or CCD camera system (a Peltier-cooled CCD camera is illustrated). Another port, located near the base at the front of the microscope, can also serve as an attachment point for a camera system (a traditional 35-millimeter camera is shown in the figure). In the figure presented above, wide-spectrum fluorescence illumination is filtered to produce a narrow bandwidth of green excitation wavelengths, which are capable of exciting specific fluorophores in the specimen. Secondary fluorescence (red light) passes back through the objective and is distributed throughout the microscope optical system. Transmitted illumination is provided by a tungsten-halogen lamphouse that is positioned on the microscope pillar, above the stage. Light from the lamphouse passes through a collector lens, a series of filters, and the field diaphragm before entering the condenser front aperture. After being focused by the condenser lens elements, transmitted illumination is projected onto the specimen, which is placed on the stage. The light that is diffracted, refracted, and not absorbed by the specimen continues through the objective and into the microscope optical train where it can be directed to the eyepieces or to a camera system. FLUOROCHROME: A natural or synthetic dye or molecule that is capable of exhibiting fluorescence. Fluorochromes (also termed fluorescent molecules, probes, or fluorescent dyes) are usually polynuclear heterocyclic molecules containing nitrogen, sulfur, and/or oxygen with delocalized electron systems and reactive moieties that enable the compounds to be attached to a biological species. ORGANELLE PROBES: most cell organelles could be stained with specific fluorochromes. Mitochondrium: MitoTracker and MitoFluor, Lysosome: LysoTracker and LysoSensor, Golgi apparatus: BODIPY, Endoplasmic reticulum: DiOC, Blue-White DPX, DNA/nucleus: Acridine orange, Propidium iodide, DAPI, Hoechst IMMUNOFLUORESCENCE MICROSCOPY A targeted molecular species (protein, nucleic acid, membrane, etc.) in a specimen is labeled with a highly specific fluorescent antibody. After the labeled antibodies have been excited by a selected region of wavelengths, secondary fluorescence emission is gathered by the objective to form an image of the specimen. Antibodies are labeled either by coupling directly with a fluorochrome (fluorescent dye; termed direct immunofluorescence), or with a second fluorescent antibody that recognizes epitopes on the primary antibody (indirect immunofluorescence). FLUORESCENT PROTEINS Green Fluorescent Protein (GFP): A naturally occurring protein fluorescent probe derived from the jellyfish Aequorea victoria, which is commonly employed to determine the location, concentration, interactions, and dynamics of a target protein in living cells and tissues. The excitation and emission spectra of enhanced GFP (a genetic derivative) have maxima at 489 nanometers and 508 nanometers, respectively. In order to incorporate the GFP (or any of its genetic derivatives) into a cell, the DNA sequence for the gene is ligated to the DNA encoding the protein of interest. After cultured cells have been transfected with the modified DNA, they are able to express chimeric fluorescent proteins for observation in the microscope. There are genetically modified variants of GFP such as blue fluorescent protein (BFP), cyan fluorescent protein (CFP), yellow fluorescent protein (YFP) CONFOCAL LASER SCANNING MICROSCOPY: A popular mode of optical microscopy in which a focused laser beam is scanned laterally along the x and y axes of a specimen in a raster pattern. The emitted fluorescence (reflected light signal) is sensed by a photomultiplier tube and displayed in pixels on a computer monitor. Signal photons that are emitted away from the focal plane are blocked by a pinhole aperture located in a plane confocal with the specimen. This technique enables the specimen to be optically sectioned along the z axis (Z-sectioning). 3D Reconstruction After Z-sectioning, the computer attached to the confocal microscope can generate virtual images of the specimen what can be viewed from any desired angles. 4D imaging The confocal microscope can be programmed to take pictures of the living sample at any desired time intervals. The resulting pictures are put together as movie files. FLUORESCENCE RESONANCE ENERGY TRANSFER (FRET): An adaptation of the resonance energy transfer phenomenon to fluorescence microscopy in order to obtain quantitative temporal and spatial information about the binding and interaction of proteins, lipids, enzymes, and nucleic acids in living cells. One of the proteins is fused to CFP, the other is fused to YFP. If there is interaction between the two proteins, we get yellow signal after exciting CFP, if there is no interaction between the two proteins, we get blue signal after exciting CFP. In case of interaction, the light emitted by CFP excites YFP. FLUORESCENCE IN SITU HYBRIDIZATION (FISH): The fluorescence FISH technique is based on hybridization between target sequences of chromosomal DNA with fluorescently labeled single-stranded complementary sequences (termed cDNA) to ascertain the location of specific genetic sequences. In medical practice, the most important application of FISH is the prenatal diagnosis of chromosome number abnormalities (and other chromosomal mutations). ELECTRON MICROSCOPY Electron microscopy is taught in detail by physics and anatomy. It uses high energy electron rays for imaging. Two versions exist: transmission and scanning electron microscopy.