Technological advances in microscopes
advertisement

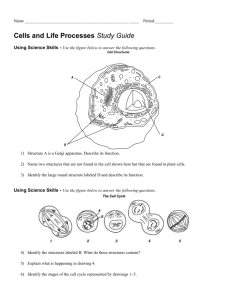
Technological Advances in Microscopes Researchers wanted to study living cells in the tiniest molecular detail. In 1873, Ernst Abbe showed a physical limit for the maximum resolution of traditional optical microscopes. The resolution it could never be better than 0.2 micrometers. Compound Light Microscopes Uses light Has two lenses Magnification limited Electron Microscope Developed in the 1930s the electron microscope allowed for higher magnification used electron beams (instead of light) and focused with an electromagnet (no lenses) the light microscope produces magnifications up to 2000X the electron microscope produces images that are magnified up to 50 000X or higher The electron microscope allowed scientists to see better quality images at higher Magnification Scanning Electron Microscope (SEM) Electrons are reflected from the surface of the specimen Produces a 3-D image Good for the thicker specimens Lacks the magnification and resolution of the transmission electron microscope 19 Transmission Electron Microscope (TEM) Uses beams of electrons Magnification of 2 000 000x Has two limitations: Good only for thin specimens Only dead cells can be observed Fluorescence The development of dyes was very useful to study the parts of cells. But these required the absorption of light which provide problems with measurement. Fluorescent dyes overcome this problem because we just measure the intensity of the emitted light. The intensity is directly proportional to the amount of excited dye. Epiflourescence is useful because most of the excitation light is transmitted through the specimen. Only reflected excitatory light reaches the objective together with the emitted light and the epifluorescence method therefore gives a high signal-to-noise ratio. Schematic of a fluorescent microscope. https://en.wikipedia.org/wiki/Fluorescence_microscope#/media/File:FluorescenceFilters_2008-09-28.svg CC BY-SA 3.0 Source of fluorescence There are several methods of creating a fluorescent sample; the main techniques are labeling with fluorescent stains or, in the case of biological samples, expression of a fluorescent protein. Alternatively the natural fluorescence of a sample (i.e.,autofluorescence) can be used. In the life sciences fluorescence microscopy is a powerful tool which allows the specific and sensitive staining of a specimen in order to detect the distribution of proteins or other molecules of interest. As a result there is a diverse range of techniques for fluorescent staining of biological samples. 20 Biological fluorescent stains Many fluorescent stains have been designed for a range of biological molecules. Some of these are small molecules which are fluorescent and bind a biological molecule of interest. Major examples of these are nucleic acid stains which bind the minor groove of DNA, thus labeling the nuclei of cells. Other fluorescent stains are drugs or toxins which bind specific cellular structures and have been made with a fluorescent reporter. A major example of this class of fluorescent stain is phalloidin which is used to stain actin fibres in mammalian cells. There are many fluorescent molecules called fluorophores or fluorochromes which can be chemically linked to a different molecule which binds the target of interest within the sample. Eg. Fluorescently labeled antibodies. Immunofluorescence This technique uses the highly specific binding of an antibody to its antigen in order to label specific proteins or other molecules within the cell. A sample is treated with a primary antibody specific for the molecule of interest. A fluorophore can be directly conjugated to the primary antibody. Alternatively a secondary antibody, conjugated to a fluorophore, which binds specifically to the first antibody can be used. Indirect immuno-cytochemistry This detection method is very sensitive because the primary antibody is recognized by many molecules of the secondary antibody. The secondary antibody is linked to a marker molecule that makes it detectable. 21 Molecular structure of green fluorescent protein GFP Stimulated emission depletion (STED) microscopy, developed by Stefan Hell in 2000. Two laser beams are used; one stimulates fluorescent molecules to glow, another cancels out all fluorescence except for that in a nanometre-sized volume. Scanning over the sample, nanometre for nanometre, gives an image with a resolution better than Abbe's limit. 22 Eric Betzig and William Moerner worked on single-molecule microscopy. The fluorescence of individual molecules are turned on and off. Scientists image the same area multiple times, letting just a few molecules glow each time. Adding these images gives a dense super-image resolved at the nanolevel. In 2006 Betzig used this method for the first time. The following image is a 3-D version of PALM showing microtubules in a cell from a fruit fly. The tubules are labeled for depth, with red lower and blue and violet higher. A conventional optical microscopy image of the same cell (left) is shown for comparison. Credit: Jim and Cathy Galbraith, Gleb Shtengel, Harald Hess, HHMI/Janelia Research Campus. There are still some problems, such as capturing good images of cellular parts in motion and removing optical errors caused by the deep three-dimensional environment of the cell. But super-resolution techniques are revolutionizing our understanding of cell biology. 23