Notes Packet Water
advertisement

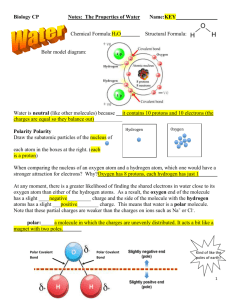
Biology CP Notes: The Properties of Water Chemical Formula: Name: Structural Formula: Bohr model diagram: Water is neutral (like other molecules) because Polarity Draw the subatomic particles of the nucleus of each atom in the boxes at the right. Hydrogen Oxygen When comparing the nucleus of an oxygen atom and a hydrogen atom, which one would have a stronger attraction for electrons? Why? At any moment, there is a greater likelihood of finding the shared electrons in water close to its oxygen atom than either of the hydrogen atoms. As a result, the oxygen end of the molecule has a slight _____________ charge and the side of the molecule with the hydrogen atoms has a slight ___________ charge. This means that water is a polar molecule. Note that these partial charges are weaker than the charges on ions such as Na+ or Cl-. polar: Polar Covalent Bond Polar Covalent Bond Kind of like the poles of earth! 1 Hydrogen Bonding Polar molecules (such as water) can attract each other because of their partial (+) and (-) charges. ____________________________: the attractive force between the hydrogen in a polar molecule and the oxygen or nitrogen in another polar molecule Hydrogen bonds are weaker than covalent or ionic bonds. Water is able to form multiple hydrogen bonds, which account for many of water’s special properties. How many hydrogen bonds Are shown in the picture to the right? Hint: look carefully! Water’s special properties 1. : An attraction between molecules of the same substance. This causes water molecules to be drawn together. Examples: 2. : An attraction between molecules of different substances. Examples: 3. : The amount of heat energy required to raise the temperature of a substance. Water’s heat capacity is unusually high. Examples: 2 4. : The amount of mass per unit volume. (D = m / v). Solid water (ice) has a lower density than liquid water. Examples: 5. Water is known as the ______________________________: In reality, water can’t dissolve everything…but water is an excellent solvent, and it’s especially common in and around living things. Solution Solvent Solute What are some substances that water can easily dissolve? Water’s polarity gives it the ability to act as an excellent solvent because it can dissolve both ______________ compounds AND other ______________ molecules. With polar solutes (like sugar), water molecules surround and separate the polar molecules from each other. With ionic solutes (like salt), water molecules surround and separate the individual ions from each other, a process known as dissociation. How Salt Dissolves in Water 3 2.2 Properties of Water The Water Molecule For Questions 1–4, write True or False on the line provided. 1. Water is a polar molecule. 2. Hydrogen bonds are an example of adhesion. 3. Covalent bonds give water a low heat capacity. 4. A hydrogen bond is stronger than a covalent bond. Write your answers in the spaces provided. 5. Compared to most other substances, a great deal of heat is needed to raise the temperature of water by a given amount. This is because water…. 6. Explain the distribution of partial charges in a water molecule. 7. Explain the difference between cohesion and adhesion. 8. Which is more dense – ice or water? How do you know? 9. Explain the difference between how water dissolves a sugar vs. a salt. 10. Explain the difference between the following terms: Hydrogen Bond and Covalent Bond 4