Unit:
advertisement

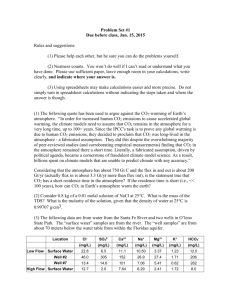
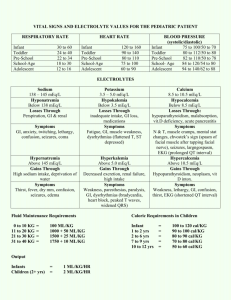
Unit: Electrolytes I - (Na, K, Cl, CO , Li, and anion gap) 2 7elect.wpd Tasks 1. Review & recall electrolyte information presented in MLAB 2360. 2. Review classroom notes covering the following: a. flame photometry and ISE principles b. normal and abnormal concentrations of sodium, potassium, chloride, and carbon dioxide. c. chloride by coulometry principle d. principle of titration 3. Determination of Na, K, Cl, & CO2 in serum/plasma and urine 4. Review of CLB determination in CSF 6. Calculation of anion gap Objectives Upon completion of this exercise, the student will be able to: 1. Briefly explain how the internal-standard flame photometer functions. 2. Identify components of a flame photometer and discuss the purpose of each. 3. Review colorimetric procedures for determining Na and K, Cl and CO2. 4. Name three (3) common procedures for determining chloride. 5. Define and explain the principle of titration. 6. Explain the principle of coulometry and the use of the chloridometer. 7. Determine chloride content using coulometric and/or titration methods. 8. Explain manual methods most commonly employed for CO2 determinations. 9. Explain the clinical significance of high and low Na, K, Cl, and CO2 levels. 10. Explain the clinical significance of and calculate the anion gap. 11. Explain modifications of internal standards of the flame photometer for lithium determinations. 12. Identify the specimen of choice, normal values, reagents & instrumentation, normal values, and quality control requirements for Na, K, Cl, CO2 and anion gap determination. MLAB 2401- Clinical Chemistry Lab Manual C 51 13. Review learning sources to define / describe the following terms: hyper/hyponatremia, hyper/hypokalemia, hyper/hypochloremia, ion-specific electrodes, coulometry, chloridometer, amperometric, and titration . Supplies and Equipment 1. Sodium and Potassium - To be announced 2. Chloride B (chloridometer) 3. 4. a. Acid buffer (ASP #B5968-5A) d. Chloride meter b. Beakers 100 μl pipet c. ClB standard solution e. f. Silver polish Chloride B (Sigma #461) a. Chloride reagent & standard(s) b. Spectrophotometer capable of measuring absorbance at 460 nm. c. Cuvets with optical properties suitable for use at 460 nm. d. Pipeting devices for the accurate delivery of volumes for the assay. Carbon Dioxide B (Sigma #131-UV) a. Spectrophotometer with temperature controlled compartment capable of measuring absorbance at 380 nm. b. Cuvets suitable for use at 380 nm. c. Pipeting devices for the accurate delivery of volumes required for the assay. d. Timer e. Carbonate/Chloride Combined Standard, Catalog No. 955-30 General Information 1. Approximate Serum Electrolyte Concentrations and Electrical Neutrality Cations Na+ Anions 142 mEq/L ClB 103 mEq/L K+ 4 mEq/L HCOB3 27 mEq/L CA2+ 5 mEq/L HPO42- 2 mEq/L Mg2+ 2 mEq/L SO42- 1 mEq/L C 52 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) others 1 mEq/L organic acids Total 5 mEq/L 154 mEq/L proteins 16 mEq/L Total 2. 154 mEq/L Normal and Panic Values Panic Values Serum Reference Analyte Range Low High Urine Na+ 135-148 mEq/L 130 mEq/L 155 mEq/L 40-220 mEq/24 hr 4. K+ 3.5-5.3 mEq/L ClB 98-106 mEq/L CO2 24-30 mmol/L 3.0 mEq/L 6.0 mEq/L 25-125 mEq/24 hr Urine Calculation (24 hour specimen) Note: Depending on the concentration of the specimen, a urine sample for Na/K analysis may need to be diluted (often x 20 dilution is adequate). Readout in mEq/L x dilution factor x # of liters in the 24 hour collection = mEq/24 hr. Clinical Significance 1. Na+, K+, ClB ,& CO2 B Please refer to the chemistry lecture guide & textbook. for additional information on these electrolytes and associated terms. Additional information on CO2 is available in the pH/ABG lab in this manual. 2. Li B Although it can be found naturally in drinking water, the electrolyte / mineral lithium is not normally found in serum in detectable amounts. Lithium carbonate salts are used to treat and prevent manic depression. Lithium levels are monitored to avoid toxicity. Therapeutic range = 0.5-1.5 mmol/L (mEq/L). Toxicity may occur at levels >1.5, while little therapeutic value is obtained at low levels. MLAB 2401- Clinical Chemistry Lab Manual C 53 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Methods of Determination 1. Sodium (Na+) and Potassium (K+) a. Emission flame photometry Solutions containing alkali ions diluted with an internal standard (usually lithium or cesium) are aspirated into the flame. The atoms emit light at one or more distinctive wavelength: Na+ B 589 nm K+ B 768 nm Li+ B 761 nm Cs+ B 852 nm The light emissions are measured directly or indirectly. Since the emission energy varies with flame temperature and other conditions, there is a need to monitor these variations with an internal standard. Lithium (or cesium) solution is continuously being aspirated along with the sodium and potassium in the patient sample, control sera, or Na/K standard. Changes in emission energy of the sodium/potassium due to flame temperature variation, aspiration rate, etc. would also occur to the emission energy of the lithium, therefore the concentrations of Na+ and K+ are determined by comparing their emission ratios with that of the Li internal standard. High Li concentration in the diluting fluid prevents interference in determination of K levels from transfer of energy from Na to K during excitation. Review of the principle of flame photometry. 1. Outer shell electrons of Na, K, Li, Cs, etc. expand their orbits when heated. 2. As they cool, returning to ground state, the energy is given off in the form of light. 3. The wavelength of light is characteristic for the particular element. 4. The intensity of the light is proportional to the amount of the element being measured. C 54 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) 5. An internal standard is added to the diluent to compensate for variation in flame temperature, flow rate, etc. b. Atomic Absorption Flame Spectrophotometry Measures the amount of energy absorbed by Na and K rather than emitted. This technique is more sensitive but less convenient than flame photometry. Review of the principle of atomic absorption photometry. 1. A hot flame releases metallic atoms from molecules. 2. The ground state atoms will absorb monochromatic light generated by a hollow cathode tube containing the element being measured. 3. The amount of monochromatic light being absorbed (by the specimen element) is proportional its concentration. c. d. Colorimetric Assays Using Precipitation and Isolation as Complex Salts (obsolete) 1) Na B precipitate as sodium uranyl acetate 2) K B precipitate as potassium, sodium-cobalnitrate Electrochemical Measurement using Ion-Specific Electrodes (ISE) B (Ion-Selective Electrodes) Newest technique used in automated equipment. Refer to Instrumentation IV, section on Ion-Selective Electrodes for specifics on sodium and potassium measurement by ISE. e. Approaches by Discrete Clinical Analyzers - The DuPont ACA , Beckman ASTRA, Biomedical NOVA 4+4, Kodac Ektachem and many other modern analyzers quantitatively measure electrolytes in serum, heparinized plasma, and pre-diluted urine by ISEs. These instruments may also provide for the calculation of anion gap. 2. Chloride Ion (ClB) Methods of chloride determination. a. Titration methods MLAB 2401- Clinical Chemistry Lab Manual C 55 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Whitehorn Titration Method B The chloride present in a protein-free filtrate is precipitated as silver nitrate solution. The surplus silver nitrate is then titrated with thiocyanate using ferric ammonium sulfate as the indicator. Schales and Schales Titration Method B The sample is titrated with a standardized mercuric nitrate solution in the presence of diphenylcarbazone as indicator. Chloride ions (ClB) in the sample combine with mercuric ions (Hg++) to form mercuric chloride (HgCl2), a colorless, soluble, but only slightly ionized compound. As long as chloride ions are present, mercuric ions preferentially react with them. At the end point, when all chloride ions have been complexed, the excess mercuric ions produce a violet color in the presence of diphenylcarbazone. Hg++ + 2 ClB HgCl2 Hg++ + Diphenylcarbazone violet color Bilirubin or hemoglobin may obscure the endpoint, therefore, protein-free filtrates should be prepared from icteric or hemolyzed specimens. Information on titration: *Titration is a method of quantitative analysis. It is a method of measuring the concentration of one solution by comparing it with a measured volume of a solution whose concentration is known. The mathematical equation (V1C1 = V2C2) is used to convert solutions of one normality to another. So, if the concentration of a solution is unknown, it can be found by measuring the volume of the unknown solution that will read with a measured amount of a solution of known concentration (called a standard solution). This process is known as titration. When titration is used to determine concentration, the concentration is traditionally expressed in terms of normality or equivalents. Normality is employed because it is a unit that provides a basis for direct comparison of strength for all solutions. Remember that normality is the number of gram equivalents per liter of solution. A gram equivalent is the amount of a compound that will liberate, combine with, or replace one gram atom of hydrogen. Therefore, one equivalent of a compound will react with exactly one equivalent of any other compound. For example, one equivalent of any acid will exactly neutralize one equivalent of any base. C 56 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) In any titration procedure, certain things must be present: 1) A standard solution of known concentration. 2) An accurately measured volume of standard solution or unknown. 3) An indicator to show when the reaction has reached completion. 4) A buret to measure volume of solution required to reach the end point. The point at which equal concentrations of the standard and the unknown are present is called the end point of the titration. Various means of detecting the end point are used. Sometimes the formation of a precipitate indicates that the end point has been reached. A change in color of one of the reacting solutions can also indicate the end point. The common method is through the use of an indicator solution which is a third solution added in the titration procedure (in addition to the standard and unknown). The procedure for chloride determination based on the method of Schales and Schales incorporates a mercuric titration using diphenylcarbazone as indicator. b. Coulometric titration - Chloridometer principle B When a constant direct current is applied to silver generator electrodes, a constant flow of silver ions is released into the solution. The silver ions combine with chloride in the sample to form silver chloride precipitate. When all chloride has been precipitated, free silver ions cause an increase in current which then stops a timer. The chloride concentration is determined as a function of time. c. Colorimetric procedures - Colorimetric procedures for determination of chloride are relatively simple and often adaptable to automation. Autoanalyzer Method B The specimen is mixed with a solution of mercuric thiocyanate. As a result of the high affinity of chloride for mercury ions, undissociated mercuric chloride is formed, resulting in the release of free thiocyanate. This reacts with the iron MLAB 2401- Clinical Chemistry Lab Manual C 57 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) in the ferric nitrate reagent to form the highly colored compound which is measured photometrically. This method is used by the ACA. Manual Method - The Sigma Diagnostics Chloride method is based upon the quantitative displacement of thiocyanate by chloride from mercuric thiocyanate. The liberated thiocyanate forms a red complex with ferric ions with an absorbance maximum at 460 nm. The color intensity at 460 nm is directly proportional to chloride concentration in the sample. 3. Carbon Dioxide (CO2) Total CO2 content is a measure of the bicarbonate and the dissolved CO2 and carbonic acid. a. Manometric/Gasometric Analysis Method B The Natelson microgasometer is used. Carbon dioxide is liberated from an aqueous solution by lactic acid. Under reduced pressure, the CO2 is shaken out, brought to constant volume and the pressure measured. The carbon dioxide is then absorbed into sodium hydroxide, the residual gases are brought to the same constant volume and the second pressure measured. The drop in pressure in mm can be multiplied by factors to bring the results to volumes percent or mEq/L. b. Van Slyke-Cullen Method B Acid is added to plasma and the CO2 produced in this reaction is liberated by means of a partial vacuum and measured. Caprylic alcohol is used to prevent foaming. c. Colorimetric & Enzymatic methods of carbon dioxide measurement are available. Sigma Diagnostics #131 is an enzymatic endpoint reaction measured in the ultraviolet wavelength range. 4. Anion Gap C 58 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) a. The anion gap is a mathematical approximation of the difference between the unmeasured anions and cations in serum or plasma. About 2/3 of a normal anion gap is caused by plasma proteins, and the remaining 1/3 by phosphate and sulfate ions and organic acids. b. Clinical Significance B The anion gap is useful in differentiating or monitoring certain conditions of metabolic acidosis and metabolic alkalosis. c. Calculations (two options); and Normal Values: 1) Na + K - (Cl + HCO3B) NV 7-14 mEq 2) Na - (Cl + HCO3B) NV 8-12 mEq d. e. Causes of an Increased Anion Gap 1) Decreased unmeasured cations 2) Increased unmeasured anions Causes of a Decreased Anion Gap Although a true decreased anion gap rarely occurs, it has been noted in some multiple myeloma patients. Possibility of instrument error should be eliminated before reporting a decreased anion gap. Procedures ClB Labconco Digital Chloridometer 1. Obtain chloride standard, controls, and unknowns and warm them to room temperature. 2. Thoroughly clean all four electrodes with silver polish, rinse with distilled water and buff with tissue. Be certain no residue remains between the indicator electrodes at their common mounting post. Avoid getting skin oils on the electrodes. MLAB 2401- Clinical Chemistry Lab Manual C 59 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) 3. After cleaning, place a vial filled with @ 4 mL of Chloridometer Acid Reagent (or 4 mL of acid solution and 4 drops of gelatin reagent) in the vial holder. Set the RANGE switch to LOW, the TITRATION switch to AUTO and raise the holder so that the electrodes are immersed and the stirrer begins. 4. If a reading does not appear after 30 seconds, re-rinse the electrodes and re-titrate using a fresh vial. Do this until a reading is obtained. 5. Place RANGE switch in the HIGH position. 6. Pipet 100 μL of the 100 mEq/L standard into the acid reagent. The addition of a sample with a chloride concentration greater than 30 mEq/L will automatically reset the display and begin the titration. If the titration endpoint displayed is 100 mEq/L 2 mEq, continue with unknowns. If standard's concentration endpoint is unsatisfactory after several attempts, contact instructor. 7. To analyze controls and unknowns, pipet 100 μL of sample into the acid reagent after the endpoint is reached and the number is recorded, a new sample may be added. Analyze in duplicate, and record results. 8. As the solution becomes whiter with precipitated silver chloride, the delay before a titration begins will increase until a titration cannot be initiated. May need to replace solution after 10-15 analysis when using 100 microliter size sample. 9. Lower the vial, replace with fresh vial and, after raising the new vial, proceed as described in step c above. 10. For each new vial, the titration procedures is the same. The electrodes only need to be cleaned if there are visible deposits. When the instrument is not in use, leave the electrodes immersed in distilled water and set the TITRATION switch to STANDBY or the RANGE switch to POWER OFF. Procedure : Chloride (Sigma #461) colorimetric C 60 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Principle See product insert. Specimen Serum or heparinized plasma. Promptly separate specimen from cells or clot to prevent shifts in ionic equilibria. Chloride in serum or heparinized plasma is stable for 1 week refrigerated or frozen. Reagents See product literature for contents of Sigma Diagnostics Chloride Reagent . DANGER: Reagent may be fatal or cause blindness if swallowed. Vapor harmful. Combustible. Wear suitable protective clothing, gloves and eye / face protection. In case of contact with eyes, flush with large volume of water and seek medical advise. See product insert for additional information. Deterioration: Chloride Reagent is not suitable for use its absorbance at 460 nm is more than 0.300. Manual Procedure 1. Set spectrophotometer wavelength at 460 nm, temperature to the assay temperature (ambient), and the absorbance reading to zero with water as reference. NOTE: Assay temperature can range from ambient / RT to 37C as long as the temperature of the reagent should be the same for all tubes. 2. Label tubes for REAGENT BLANK, STANDARD(S), CONTROL(S) and PATIENT SAMPLES. 3. Pipet 3.0 mL of Chloride Reagent into each tube and warm to assay temperature. 4. Pipet 25 uL of water, standard and samples into their respective tubes. 5. Mix gently by inversion and incubate for 5 minutes. (NOTE: The color is stable for at least 1 hour provided the temperature of the reaction mixture remains the same.) MLAB 2401- Clinical Chemistry Lab Manual C 61 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) 6. Read and record absorbance of each at 460 nm. 7. Subtract the absorbance of the BLANK from the absorbance of STANDARD(S), CONTROL(S) and PATIENT SAMPLES the to obtain change in absorbance due to chloride content (A Standard, A Specimen). Calculations Determine the Chloride content of the sample as follows. A SAMPLE Chloride (mEq/L) of sample = - A BLANK ______________ A STANDARD - X Concentration of Standard A BLANK Limitations Procedure is linear from 50 to 130 mEq/L. If sample exceeds linearity, dilute with deionized water, repeat assay and multiply result by dilution factor. Expected Values The expected range of chloride concentration was determined to be from 97 - 106 mEq/L. This range is similar to reference values reported in the literature. It is strongly recommended that each laboratory establish its own expected range characteristic for the population of the local area. Procedure : Carbon Dioxide (Sigma #131-uv) Enzymatic Principle Phosphoenol pyruvate carboxylase (PEPC) catalyzes the reaction between phosphoenol pyruvate and carbon dioxide (bicarbonate) to form oxalacetate and phosphate ion. In the second step of the reaction, catalyzed by malate dehydrogenase (MDH), oxalacetate is reduced to malate with simultaneous oxidation of equimolar amount of reduced nicotinamide adenine dinucleotide (NADH) to C 62 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) (NAD). This results in a decrease in absorbance at 380 nm which is directly proportional to the CO2 level. Specimen Serum or heparinized plasma. Ideally, venous blood should be collected and handled anaerobically. After prompt separation from cells or clot, specimen should be kept tightly stoppered. CO2 content of blood is stable for 1 hour when stored at 2-4C under anaerobic conditions. Reagents Reagents should be reconstituted according to directions. See product literature for contents of Sigma CO2 REAGENT A, CO2 REAGENT B, and CO2 DILUENT. These reagents are for In Vitro diagnostic use only. *DANGER: They contain sodium azide which is toxic if ingested any may react with lead and copper plumbing for form highly explosive metal azides. On disposal flush with large volume of water. Avoid contact and inhalation of CO2 REAGENT B. Reagent Preparation for Sample Start Procedure (Alternate Procedure) 1. Reconstitute CO2 Reagent A with volume of deionized water indicated on vial label. 2. Reconstitute CO2 REAGENT B with volume of CO2 Diluent. Stopper vials and mix several times by gentle inversion. 3. Prepare Sample Start Reagent by adding 1 mL of CO2 Reagent B to 10 mL of CO2 REAGENT A and use immediately. Note: Avoid contamination of the reagents with CO2. Do not blow into pipets, since breath contains a high content of CO2. Do not leave bottles open unnecessarily, since CO2 from air can contaminate the reagent. Deterioration: Reagents are not suitable for use if the absorbance at 380 nm of freshly combined reagent, is less than 1.000. Manual Alternate Procedure MLAB 2401- Clinical Chemistry Lab Manual C 63 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) 1. Prepare CO2 Sample Start Reagent according to instructions. (Each bottle does 5 tests.) 2. Set spectrophotometer wavelength at 380 nm, temperature to the assay temperature (ambient), and the absorbance reading to zero with water as reference. Note: Procedure can be performed at ambient / RT, 30 or 37C temperature. 3. Label tubes for REAGENT BLANK, STANDARD, and SAMPLES. 4. Pipet 2 mL of CO2 Sample Start Reagent into each tube and warm to assay temperature. 5. Pipet 20 uL of water, standard and samples into their respective tubes. 6. Mix gently by inversion and incubate for 10 minutes. 7. Read and record absorbance of each at 380 nm. 8. Subtract the absorbance of the STANDARD and SAMPLE from the absorbance of the BLANK to obtain change in absorbance due to CO2 content. Calculations Determine the CO2 content of the sample as follows. A BLANK Carbon Dioxide (mEq/L) of sample = - A SAMPLE ____________ A BLANK Limitations C 64 MLAB 2401 - Clinical Chemistry Lab Manual - A STANDARD X Concentration of Standard UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Procedure is linear to 50 mEq/L.. Expected Values See product insert and information reported in the literature. It is strongly recommended that each laboratory establish its own expected range characteristic for the population of the local area. MLAB 2401- Clinical Chemistry Lab Manual C 65 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Name_________________________________ Date_________________________________ Electrolyte Worksheet I. Chloride Sigma # 461 Wavelength _____________ Linearity _____________ Identification Spectrophotometer Used ______________________ Absorbance Abs test - Abs blank Concentration (units) Blank Standard ____________ Standard ____________ Control 1 ____________ Control 2 ____________ Calculation formula(s) and examples Quality Control Your Results Controls= range of expected results. In control? Yes / No Level 1 ID______________ Level 2ID_______________ C 66 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Quality Control Accepting Patient Results? II. Reason Chloride by Chloridometer (NOTE: Work to obtain results that check within 2 mEq.) A. Instrument set-up/Standardization B. Measurements (in duplicate) Sample Lot #/ID # ClB Standard Control I (expected values (ClB) mEq/L 1) 2) avg. 1) 2) avg. 1) 2) avg. 1) 2) avg. 1) 2) avg. 1) 2) avg. ) Control II (expected values ) Unknowns III. Patient ID & Specimen Type Carbon Dioxide Procedure Instrument Wavelength Sample Lot #/ID # Abs. 380 nm (CO2), mEq/L Standard Control I (expected values ) Control II (expected values ) MLAB 2401- Clinical Chemistry Lab Manual C 67 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Unknowns Patient ID & Specimen Type Calculations: Quality Control Your Results Controls= range of expected In control? Yes / results. No Level 1 ID______________ Level 2ID_______________ Accepting Patient Results? IV. Reason Anion Gap A. Formula for calculations: B. Anion Gap Normal Values: C. Using the information provided, calculate the AG for the following patient. Patient 1: Na = 140 mEq/L, K = 5.1 mEq/L, Cl = 105 mEq/L, CO2 = 28 mEq/L D. Calculations C 68 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) Name Date_________________________________ Study Questions Instructions: Legibly write your answers in the space provided. Unless otherwise indicated, each question is worth one point. Using lecture notes, reading assignments and information presented in this lab, answer the following questions. 1. How could poor collection/handling process affect the electrolyte results? 2. Using flame photometry methodology, what is the relationship between the intensity of color (wavelength) produced and the amount of element being measured? 3. What is the purpose of the lithium diluent? 4. What other compound can be used in place of lithium as an internal standard in flame photometry? 4. What is the body's major intracellular cation? 5. What is the body's major extracellular cation? MLAB 2401- Clinical Chemistry Lab Manual C 69 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) 6. Which plasma electrolyte, in high or low concentrations, may cause cardiac arrest? List two causes of increased and decreased plasma concentrations of this electrolyte. (2 points) 7. According to your readings in this lab, define titration. 8. List the four things that must be present to perform a titration procedure. (2 points) 9. What equation is used to convert solutions of one normality to another? 10. Chloride is the major intracellular/extracellular anion. (circle one) 11. Briefly describe the anion gap. Include what causes it and how it is used. (3 points) 12. Calculate the following anion gaps. sodium 142 137 potassium 4.0 3.8 chloride 105 99 carbon dioxide 28 32 anion gap C 70 MLAB 2401 - Clinical Chemistry Lab Manual UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) For your own study / review CASE STUDY: Electrolytes A medical laboratory technician obtained the following results on a routine renal profile. Sodium: 139 mEq/L Potassium: 4.1 mEq/L Chloride: 118 mEq/L CO2: 20 mmol/L BUN: 19 mg/dL Creatinine: 0.9 mg/dL Glucose: 250 mg/dL The sodium, potassium, and CO2 are measured by ion selective electrodes. The chloride is measured by the ferric thiocyanate method. Upon reviewing the results, the technician performs a quick written calculation and decides that the specimen should be re-analyzed, believing that the results are not valid. 1. Which of the above results seem unusual? 2. Provide the simple calculation that can be used to check the validity of these results. 3. Calculate the anion gap. 4. State the normal values for the anion gap. 5. What are some possible causes of an increased abnormal anion gap value? 6. What are some possible causes of a decreased anion gap value? 7. How can the anion gap be used to evaluate the validity of electrolyte analysis? CASE STUDY: Electrolytes and Water Balance A 58 year old woman with a history of hypertension arrived in the ER complaining of nausea, oliguria, and vomiting. The following are electrolyte results obtained. Sodium: 115 mmol/L Potassium: 3.5 mmol/L Chloride: 68 mmol/L The patient was regularly taking medication to control the hypertension. MLAB 2401- Clinical Chemistry Lab Manual C 71 UNIT: Electrolytes I (Na, K, Cl, CO2 Li, and anion gap (continued) 1. Evaluate the electrolytes for abnormalities. 2. What treatment might correct the situation? C 72 MLAB 2401 - Clinical Chemistry Lab Manual