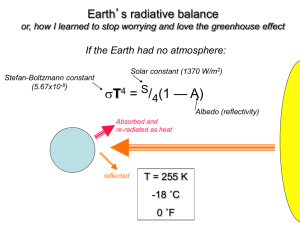
Problem Set #1
advertisement
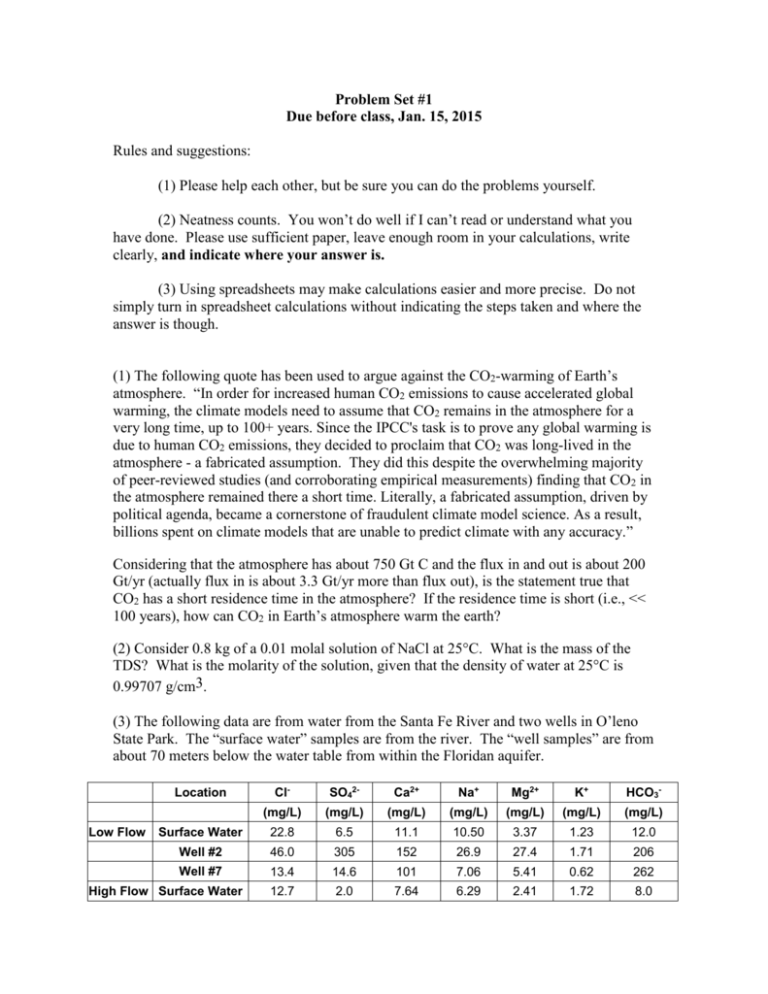
Problem Set #1 Due before class, Jan. 15, 2015 Rules and suggestions: (1) Please help each other, but be sure you can do the problems yourself. (2) Neatness counts. You won’t do well if I can’t read or understand what you have done. Please use sufficient paper, leave enough room in your calculations, write clearly, and indicate where your answer is. (3) Using spreadsheets may make calculations easier and more precise. Do not simply turn in spreadsheet calculations without indicating the steps taken and where the answer is though. (1) The following quote has been used to argue against the CO2-warming of Earth’s atmosphere. “In order for increased human CO2 emissions to cause accelerated global warming, the climate models need to assume that CO2 remains in the atmosphere for a very long time, up to 100+ years. Since the IPCC's task is to prove any global warming is due to human CO2 emissions, they decided to proclaim that CO2 was long-lived in the atmosphere - a fabricated assumption. They did this despite the overwhelming majority of peer-reviewed studies (and corroborating empirical measurements) finding that CO2 in the atmosphere remained there a short time. Literally, a fabricated assumption, driven by political agenda, became a cornerstone of fraudulent climate model science. As a result, billions spent on climate models that are unable to predict climate with any accuracy.” Considering that the atmosphere has about 750 Gt C and the flux in and out is about 200 Gt/yr (actually flux in is about 3.3 Gt/yr more than flux out), is the statement true that CO2 has a short residence time in the atmosphere? If the residence time is short (i.e., << 100 years), how can CO2 in Earth’s atmosphere warm the earth? (2) Consider 0.8 kg of a 0.01 molal solution of NaCl at 25°C. What is the mass of the TDS? What is the molarity of the solution, given that the density of water at 25°C is 0.99707 g/cm3. (3) The following data are from water from the Santa Fe River and two wells in O’leno State Park. The “surface water” samples are from the river. The “well samples” are from about 70 meters below the water table from within the Floridan aquifer. Cl- SO42- Ca2+ Na+ Mg2+ K+ HCO3- (mg/L) (mg/L) (mg/L) (mg/L) (mg/L) (mg/L) (mg/L) Surface Water 22.8 6.5 11.1 10.50 3.37 1.23 12.0 Well #2 46.0 305 152 26.9 27.4 1.71 206 Well #7 13.4 14.6 101 7.06 5.41 0.62 262 12.7 2.0 7.64 6.29 2.41 1.72 8.0 Location Low Flow High Flow Surface Water Well #2 21.3 114.0 79.10 14.10 13.80 1.43 102 Well #7 13.1 17.7 87.20 7.53 5.78 0.57 196 Please show your work for the following calculations: (a) Estimate the values for TDS of the water samples. Why are these calculations only estimates? How good do you think the estimates are? Why are the well TDS values higher than the surface water? (b) Convert concentrations from weight to molar units. (c) Convert the concentrations to meq/l. (d) Calculate the charge balance error for each analysis. Evaluate how good the analyses are and discuss possible reasons why there are errors. (e) Plot the data using meq/L units on the attached Piper diagram