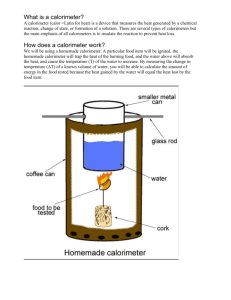
Determining the Caloric Content of Foods
advertisement

Determining the Caloric Content of Foods Introduction: Students are familiar with the Calorie content of foods. In this lab students experimentally measure the calorie content of some foods. This provides an understanding of how calorie content of foods can be determined by experiment. This lab also provides an opportunity to explain the difference between the food Calorie and the chemical calorie. To compare experimental values with those on the package, we recommend using only freeze dried samples. Water, when burned, shows a loss in mass with no resulting heat increase; varying amounts of water in food products lead to inconsistent results. Freeze drying samples provides a greater consistency. We also recommend that the calorimeter be covered on the sides and top with the insulating sleeve and cap to minimize heat loss. We also suggest that the water not be stirred to show the maximum temperature increase. After the sample is consumed, continue vacuuming until the temperature peaks; it could take several minutes. Purpose: The purpose of this laboratory activity is to measure the calorie content of a food. This value may be compared to that of other food types, or it may be compared to the value shown on the food’s packaging. Materials: food calorimeter oxygen tank and connectors vacuum pump or water aspirator 6 V 2 A (ac or dc) power supply 1-L graduated cylinder food sample large ring stand clamps safety shield analytical balance Safety: Use a safety shield around the food calorimeter. Turn on the oxygen carefully to maintain a gentle flow of oxygen. Extinguish all flames in the laboratory. Always wear safety goggles and an apron in the laboratory. Never eat or drink in the laboratory. Procedure: 1. Clamp the heat resistant platform to a ring stand at a height that permits it to be lowered, in order to give access beneath the glass vessel. 2. Secure the glass vessel by a large clamp immediately above the platform. Be sure the rubber bung (stopper) around the copper helix is tight. 3. Fill a 1 L graduated cylinder with distilled water. Use this water to fill the calorimeter to just cover the upper coil of the copper helix. Determine the volume by difference. Based on room temperature, use the density of the water to find the mass of the water in the calorimeter. 4. Place the stirrer and plastic lid in position. 5. If required, cut a small ring from rubber tubing and place this ring on the temperature probe or thermometer so that the tip or bulb is just above the top of the inner calorimeter vessel. 6. Connect the vacuum source to the outlet of the copper helix. 7. Connect the tube below the heat resistant base to the oxygen tank. 8. Adjust the tank and vacuum so that a gentle stream of oxygen can pass in to the combustion chamber. (20 kN/cm2) Adjust the oxygen stream with a pinch clamp on the hose. 9. Place the nickel crucible on the heat resistant platform so that ignition spiral can be lowered into it to touch the test food. 10. Connect the terminals to a 6V 2A power supply. Be careful not to exceed 6 volts as the ingniter will break. If in doubt, check the power supply output with a voltmeter. 11. Mass the empty nickel crucible to the nearest thousandth of a gram. Mass again with the food sample inside. 12. Place the crucible in position on the heat resistant platform. 13. Place the safety shield around the apparatus. 14. Apply a moderate vacuum. Turn on the oxygen supply SLOWLY. 15. Agitate the water until a steady temperature is reached. Record this initial temperature. 16. Lower the filament. Turn on the power supply and ignite the test substance. 17. Once ignited, raise the filament, turn it to the side, and immediately turn off the power supply. 18. The test substance should burn steadily and brightly. If sputtering occurs, decrease the oxygen supply until the food burns quietly. 19. Agitate the water continuously during combustion. 20. When the combustion is complete, turn off the oxygen supply and vacuum pump. 21. Continue to stir until a maximum temperature is reached. Record this maximum temperature. 22. Allow apparatus to cool. 23. Mass the crucible again to determine the mass of the unburned residue. 24. Record the required data in the Data Section. Data: Food tested ______________________ Volume of water ______________________ mL Mass of crucible ______________________ g Mass of food ______________________ g Mass of crucible and residue (after heating) ______________________ g Mass of food consumed ______________________ g Initial temp of water ______________________ ºC Final temp of water ______________________ ºC Temperature change _____________________ ºC Calories produced ______________________ cal Calories/ g of food ______________________ cal/g Calories per serving ______________________ Cal/ser Calculations: Determine the energy produced by the food and compare the total to the quantity shown on the package. The formula is Q=m*c*ΔT where m is the mass of water, c is the specific heat of water, and ΔT is the change in temperature of the water. 1. Determine the mass of the water inside the calorimeter in grams. a. volume*density = mass 2. Determine the specific heat of water in cal.*g-1* ºC-1. a. Specific heat of water = 1cal.*g-1* ºC-1 b. Since food energy is measured in kilocalories, specific heat = 0.001Cal.*g-1* ºC-1. 3. Determine the temperature change of the water in ºC. a. Final temp – Initial temp = Temp change 4. Determine the energy absorbed by water in Cal. a. Qw= m*c*ΔT 5. Determine the energy absorbed by the calorimeter in Cal. a. Calorimeter constant * temp change = Qc b. Qc=0.143Cal(ºC)-1 * ΔT 6. Determine the total energy produced by the burning food in Cal. a. Qw+Qc=QT 7. Determine the energy produced per gram of food in Cal./g. a. QT/mass of food burned Calculations, opt: 8. Determine the experimental Calories per serving a. Cal/g * g/serving = Cal/serving 9. Record the Calories per serving from the food package. 10. Determine the percent error. Cal / servingonpackage Cal / servingcalculated a. Percenterror 100 Cal / servingonpackage Questions: 1. Why was the calorimeter filled with water? 2. Why was the combustion chamber filled with oxygen? 3. Why was a vacuum pump used? 4. Which of the foods tested contained the highest number of calories per gram?