Q5 - Helios
advertisement

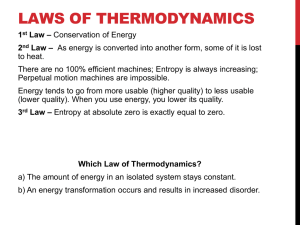
Qwizdom Grading (4% of your course grade) 1. Each correct answer = 2 points 2. Each incorrect answer or no answer = 1 3. Absent = 0 points Suppose there are 40 questions during the term. Some possibilities Perfect – all correct Always come to class Never attend class Come half the time Points 80 40 – 80 0 20 – 40 % value 4 2–4 0 1–2 3. The amount of heat necessary to raise the temperature of 1 gram of water by 1°C is referred to as the A. calorie B. kilocalorie C. British thermal unit D. joule 3. The correct answer is A, the calorie. 4. Which of the following is the smallest unit of heat energy? A. B. C. D. calorie kilocalorie Btu joule 4. 1 kcal = 1000 cal 1 kcal = 4186 J 1 cal = 4.186 J 1 Btu = 252 cal So the answer is D, the joule. From smallest to largest: J, cal, Btu, kcal 5. A chunk of ice (T = -20°C) is added to a thermally insulated container of cold water (T = 0°C). What happens in the container? A. The ice melts until thermal equilibrium is established. B. The water cools down until thermal equilibrium is established. C. Some of the water freezes and the chunk of ice gets larger. D. none of the above 5. The correct answer is C, some of the water freezes and the chunk of ice gets larger. The final temperature must be between 0 and -20°C. So more ice forms. Done with Qwizdom