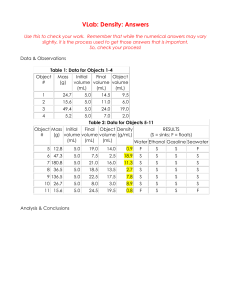
Density Problems Memory aid: “I heart density!” means D = Show
advertisement

𝑚𝑎𝑠𝑠 Density Problems Memory aid: “I heart density!” means D = 𝑣𝑜𝑙𝑢𝑚𝑒 Show work: formula; substitutions; solve–– Round appropriately. Include units during the substitution step as well as within your final answer! 1. What is the density of a piece of foam with a mass of 3.57 g and a volume of 10.89 mL? 2. A solid substance has a mass of 23.36 g and takes up a volume of 74.5 cm3. Calculate its density. 3. A piece of cement measures 3.0 cm long, 8.20 cm high, and 10.0 cm deep. It has a mass of 153 g. What is the density of the cement? 4. A rectangular eraser has a mass of 50.0 g and a volume of 200. mL. What is its density? 5. The eraser in question #4 is cut exactly in half. What is its density now? 6. It is cut exactly in half once more. What is its density now? 7. From your answers to questions #4, 5, and 6: Is it true that density depends on the the amount of a substance? Or, is it true that density is independent of the amount of substance? Explain why you chose this answer. 8. A property that depends on the amount of a substance is called an “extensive property”. [Memory aid – An extensive property depends on the “extent” (or how much) there is of something. Is density an extensive property? Explain. Answer in a complete sentence please. 9. A property that does not depend on amount, but instead depends on the composition of a material, is called an “intensive” property. Is density an intensive property? Explain your answer. 10. In order to answer questions # 4, 5, and 6 correctly, you needed to understand that as you cut something in half, both the mass and the volume would go down by ½. When two properties both decrease (or both increase) linearly, you could graph the data and see a straight line. Would it make sense to call the straight line relationship between mass and a “direct proportion” or an “indirect (inverse) proportion”? (pick one) Explain your answer. 11. The density of liquid “Substance A” is 1.97 g / cm3. The density of liquid “Substance B” is 1.89 g / cm3. Which substance will sink below the other? (A or B) Why? Show work: formula; substitutions; solve–– Round appropriately. Include units during the substitution step as well as within your final answer! 12. The density of silver is 10.5 g / mL. What is the mass of a 3.50 mL bracelet made of silver? 13. 450. grams of gasoline is spilled into a puddle of water, and the gasoline floats on top of the water. If the density of gasoline is 0.665 g / mL, what volume of gasoline is spilled? 14. Calculate the mass of 250.0 mL of a chemical whose density is 0.8765 g / mL. 15. What is the mass in milligrams of an ice cube measuring 5.80 cm by 5.80 cm by 5.80 cm, if its density is 0.917 g / mL? 16. What volume would be occupied by 0.01781 kg of Lucky Charms it their density of 0.004510 kg / mL? 17. The density of air at room temperature is 0.001200 g / cc. What would be the volume of 1.000 grams of air? 18. Using the density of air given above, what would be the mass of 1,000. cc of air at room temperature? 19. What is the density of aluminum, if 27.03 grams of aluminum displaces a volume of water equivalent to 10.00 mL? 20. Several different amounts of aluminum are used to construct a density graph with the mass in grams plotted along the y–axis and the volume in milliliters plotted along the x–axis. Using your answer to question # 19, what should the slope of this graph be? 21. Should the slope of a density graph (with mass plotted on the y-axis and volume plotted on the x-axis) be positive or negative? 22. What should the y-intercept of a density graph equal? 23. When a lava lamp is turned on, the “lava” is exposed to heat from a light bulb at the base of the lamp. The lava then rises up through the surrounding matrix. We know that mass is always conserved; but, volume is not necessarily conserved. What must be happening to the volume of the lava in order for it to float upward? Pick one –– “volume must increase”, “volume must decrease”, or “volume must be conserved (stay the same)”. 24. What must happen to the volume of the blob of lava as its sink back down again? Full sentence please. 25. There is a “density column” at the front of the classroom. Vegetable oil was poured on top of water (colored with food dye for better visibility). When an ice cube is placed on top of the vegetable oil it floats; but as the ice cube melts, droplets of water can be observed to sink down through the oil, and merge in with the colored water. Do ice cubes have a higher density or a lower density than liquid water? Explain.