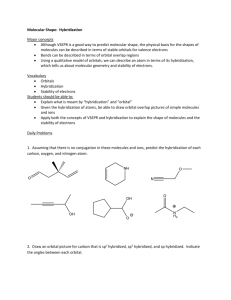
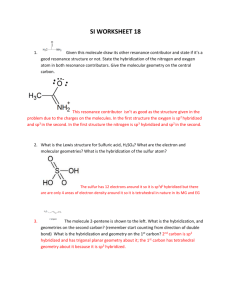
Bond Hybridization

Bond Hybridization, Sigma Bonds and Pi Bonds
Part I: Hybridization
In chemistry, hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridized orbitals are very useful in the explanation of the shape of molecules. It is an integral part of valence bond theory.
This concept proposes that since bonded atoms are not at the angles of the p orbitals, the s and p orbitals will be hybridized to match the bond angles of the attached groups.
For example, examine the energy level diagram for carbon:
2p
E
2s
1s
According to this diagram, carbon should only be able to make 2 bonds. This could result in the very unstable atom CH
2
. Scientists have found, however, that carbon can make four bonds. This is explained through bond hybridization. The s and p orbitals will combine to created new hybridized orbital with an energy level between the s and p orbitals, and the s and p electrons will place themselves into those new orbitals following regular energy level diagram rules.
E
2p
2s
1s
E
2sp 3
1s s p p p
We now have four single electrons to make four bonds with the carbon atom. Since the new hybrid bonds have used one s orbital and three p orbitals, we say that carbon has four sp 3 hybridized bonds. These bonds will spread out evenly to give us the shape of a tetrahedral.
We can do the same thing with nitrogen.
E
2p
2s
E 2sp 3
s p p p
1s
1s
We still needed to use the s and all three orbitals to create the hybridized orbitals for nitrogen, so we say that nitrogen is sp 3 hybridized. Its electron pairs will still have a tetrahedral geometry, but due to the lone pair we say that the molecule has a trigonal pyramidal geometry.
Next, try oxygen. First, complete the energy level diagram, then fill in the hybridized energy level diagram.
E
2p
2s
1s
E
1s
2sp 3
s p p p
When the orbitals in oxygen become hybridized, we need to use _____ s orbital and ______ p orbitals. This means that oxygen has _______ hybridization. The electron pairs have a
__________________ geometry, while the molecule has a ___________________ geometry.
Let’s try another atom. Take Boron, for example. Look at the energy level diagrams below.
2p
E
2p
2s
E
2sp 2
s p p
E
1s 1s
This time, we still used _____ s orbital, but only used ______ p orbitals. The third p orbital does not hybridize, and stay at its regular energy level. This means that boron has three sp2 hybridized bonds.
Now, what about those elements that can break the octet rule, such as phosphorus? Let’s look at pho sphorus’ energy level diagram and hybridized energy level diagram.
4p
4s
3p
3d
4s
3sp 3 d
3s
E
s p p p d
2p
2s
1s
2p
2s
1s
E
Phosphorus has electrons in the third energy level, so the d orbital can now come into play. To create hybridized orbitals, we use 1 s orbital, 3 p orbitals and 1 d orbital. We can say that the 5 bonds that phosphorus can make are sp 3 d hybridized. The electrons pairs have a trigonal bipyramidal geometry, while the molecule itself also has a trigonal bipyramidal geometry.
Let’s try one last atom, sulfur. Complete the energy level diagrams below:
3d
4s
3d
4s
3p 3sp 3 d 2
3s
E s p p p d d
2p
2s
1s
2p
2s
1s
Sulfur also has electrons in the third energy level, so the d orbital can now come into play again.
To create hybridized orbitals, we use ________ s orbital, _________ p orbitals and ______ d orbitals. We say that the _________ bonds that sulfur can make are ___________hybridized. The electrons pairs have an ___________________ geometry, while the molecule itself also has an
_______________________ geometry.
The following chart is a summary of what you have just learned:
Number of
Areas of
Electron
Density
Description and 3-Dimensional Shape
Two...sp
Three...sp
2
Four...sp
3
Five...sp
3 d
Six...sp
3 d 2
Part II: Single, Double and Triple Bonds
In Part one, we all of the molecules we focused on contained single bonds, which are also known as sigma bonds (
bonds). Sigma bonds are bonds formed when the orbitals overlap end to end, as in the diagram below:
Orbital overlap =
bond
Now we must wonder, what happens in an energy level diagram when there are double and triple bonds?
Consider the molecule ethene, C
2
H
4
.
Each carbon has 3 areas of electron density, so this means that each carbon sp 2 hybridized (s+p+p
= 3 areas). This means that the s orbital and 2 p orbitals have created a hybridized orbital. There is still one p orbital left. The electron in the left over p orbital will be used to create the second bond.
2p 2p
Used in 1 pi bond
E
2s
1s
E
2sp 2
1s
s p p
Used in 3 sigma bonds
The second bond created will not be an end to end bond like the first bond. It is considered a sideby-side bond, and it called a pi bond (
bond). You can see the difference between the sigma and pi bonds in the diagram below.
Now let us consider a triple bond, for example in the molecule ethyne:
Each carbon in this example has 2 areas of electron density, so it would be considered to have sp hybridized bonds (s+p = 2). The energy level diagram for one of the carbons would look like this:
Used in 2 pi bonds
E
2p
2s
E
2p
2sp
s p
1s 1s Used in 2 sigma bonds
This time the s orbital and only one p orbital form a hybridized bond, while the two extra electrons go into the higher level p orbitals. These two electrons will for two p bonds between the carbons as shown in the diagram below:
Practice Problems:
1. Determine the bond hybridization for each atom in the compound.
Formula Diagram
Type of
Hybridization
Electron Pair
Geometry around
Central Atom
CCl
4
Molecular geometry
SF
6
PBr
5
SF
4
ClF
3
XeF
2
H
2
O
2. How many sigma and pi bonds does each of the following molecules have? a) O
2 b) C
2
H
2 c) C
2
H
4 d) C
2
H
6 e) H
2
O f) C
3
H
4