EBER in situ hybridization protocol

EBER in situ hybridization protocol
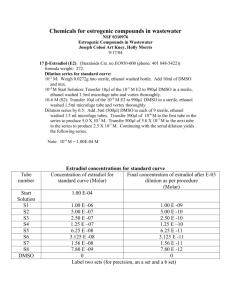
In situ hybridisation was performed using the EBER PNA-FITC probe (DakoCytomation, Denmark). Briefly slides were deparaffinized in xylene, treated with 100% ethanol and the endogenous peroxidase blocked using 1% H
2
0
2
in methanol for 20 minutes and rinsed in 96% ethanol before being air-dried.
The sections were digested with 0.1% pepsin A (Sigma) in 0.01N HCL (37 0 C for 10 minutes), washed in
RNAse free water, rinsed in 96% ethanol and air-dried. The EBER PNA probe was applied to the slides, cover-slipped, sealed and allowed to hybridise at 55 o C overnight. The slides were washed in stringent washing solution (pH10) at 55 o C for 25 minutes and then washed in 0.005% PBS Tween 20 pH7.4 and blocked with Ultra V block (Labvision).
The EBER probe was detected using rabbit anti-FITC (Dako) diluted 1 in 500 in 1%BSA/PBS for 60 minutes at room temperature. The sections were washed before incubation with biotinylated goat anti-rabbit
(polyvalent Ultravision kit, Labvision) for 10 minutes, washed and incubated with streptavidine peroxidase
(Labvision) 10 minutes. The EBER probe was detected using AEC (Zymed) for 10-15 minutes according to the manufacturer’s instructions. The sections were then washed and counterstained with haematoxylin and mounted with aquatex (Merck).
Negative controls were sections of MS tissues or EBV positive tissues from Hodgkins’ disease, nasopharyngeal carcinoma and oral hairy leukoplakia stained as above but without addition of the PNAprobe.