answers
advertisement

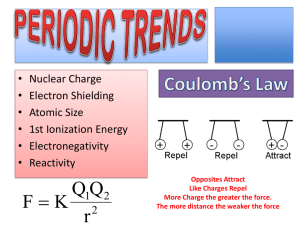
Name: _____________________________ Science 10 Modified Final Assessment Review: Chemistry Write the word from the word bank to match the definition: Valence Electrons on the outer shell of an atom Groups The columns that run up and down on a periodic table Periods The rows that run across on the periodic table Subscripts The tiny numbers behind symbols that indicate the number of atoms Neutralization The type of equation in which you add a base to a salt and end with a salt Anion A negative ion, formed when an atom gains electrons Cation A positive ion, formed when an atom loses electrons Control A variable that is kept the same in an experiment Independent A variable that is changed and tested Dependent A variable that is measured to determine the effect Reactants The starting elements on the left hand side of an equation Products The ending elements on the right hand side of an equation Litmus Strips of paper that changed red if a substance is an acid, or blue if it’s a base Indicator Any substance that changes colour to indicate the acidity or basicity of a substance Inferences Reasonable conclusions derived from observations Observations Gathering of information using the senses Synthesis/Composition Two or more substances that combine to form one new substance Decomposition A substance that is broken down into two or more substances Single Displacement One element takes the place of another element in a molecule Double Displacement The cations of two different molecules switch to places to form two new molecules Combustion A substance is burned with oxygen and often produces carbon dioxide and water OH- Bases release these ions in water H+ Acids release these ions in water Anion Composition reaction Dependent variable Independent variable Litmus Periods Single displacement reaction H+ Cation Control variable Double displacement reaction Indicator Neutralization Product Subscripts OH- Combustion reaction Decomposition reaction Groups Inference Observation Reactant Valence Electrons X-rays 1. How do you calculate the number of: a) Protons The number of protons is the same as the atomic number b) Electrons The number of electrons is the same as the number of protons c) Neutrons The number of neutrons is equal to the mass number subtract the number of protons 2. How does an element in group 17 become stable with a full valence shell of 8? Elements gain one electron 3. How does an element in group 1 become stable with a full valence shell of 8? Elements lose one electron 4. List the metalloids on the periodic table Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium. 5. List the liquids on the periodic table Mercury and Bromine 6. What are the indications that a chemical reaction has occurred? 1. A new colour appears 2. Heat, light, or sound is given off 3. Bubbles of gas are formed (new odour too) 4. A precipitate (solid material) forms in a liquid 5. The change is difficult or impossible to reverse 7. Do you think harmful cleaning products should be banned/made illegal? Various, individual answers… 8. Explain the purpose or reason behind IUPAC / Why is this organization important in Chemistry? In the early days of chemistry, there were no rules for naming compounds. They used trivial or common names but they told you little or nothing about the chemistry of the compounds. Then, in the mid- to late-1800s, chemists observed and discovered new patterns of chemical relationships. The International Union of Pure and Applied Chemistry (IUPAC) was formed in 1919 by a group of chemists. The main aim of IUPAC was to establish international standards for masses, measurements, names, and symbols used in chemistry. 9. Describe the purpose of WHMIS and why is it important. The Workplace Hazardous Materials Information System (WHMIS) is Canada's national hazard communication standard. The key elements of the system are labelling of containers of WHMIS "controlled products", making and distributing material safety data sheets (MSDSs) and worker education and training programs. Balance these chemical equations AND classify the reaction type a. Composition 1 N2 +3 H2 2 NH3 b. Composition 1 S8 + 12 O2 8 SO3 c. Decomposition 2 HgO 2 Hg + 1 O2 d. Single Replacement 1 Zn + 2 HCl 1 ZnCl2 + 1 H2 e. Composition 4 Fe + 3 O2 2 Fe2O3 f. Double Replacment 1 Fe2(SO4)3 + 6 KOH 3 K2SO4 + 2 Fe(OH)3 Name or write the formula for these Binary Ionic compounds 1. LiF Lithium Fluoride 2. lithium chloride LiCl 3. Li2O Lithium Oxide 4. lithium nitride Li3N 5. Li3P Lithium Phosphide 6. beryllium fluoride BeF2 7. BeO Beryllium Oxide 8. beryllium sulfide Be3S2 9. BF3 Boron Fluoride 10. boron chloride BCl3 Name or write the formula for these Molecular compounds 1. Sulfur hexafluoride: SF6 2. Dinitrogen oxide N2O 3. Diphosphorus pentoxide P2O5 4. Selenium dibromide: SeBr2 5. Arsenic pentafluoride: AsF5 6. CBr4 Carbon tetrabromide 7. SO3: Sulfur trioxide 8. P4O10: Tetraphosphorus decaoxide Name and/or write the formula for these Acids AND Bases a) Hydrofluoric acid Binary Acid Formula: HF b) Nitrous acid Oxoacid c) Mg(OH)2 Base Name: Magnesium Hydroxide d) Aqueous Hydrogen Bromide Binary Acid Formula: HBr e) HCl Binary Acid Classical Name: Hydrochloric Acid f) Aqueous Hydrogen Phosphate Binary Acid g) HI Binary Acid IUPAC Name: Aqueous Hydrogen Iodide h) Calcium Hydroxide Base Formula: Ca(OH)2