Solubility Curve Lab
advertisement

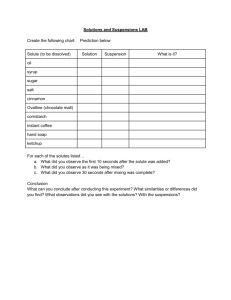
Solubility Curve of a Mystery Solute Background Solutions are homogeneous mixtures of solvents (the substance doing the dissolving-in this lab-water) and solutes (the substance being dissolved-in this lab-the unknown salt). When a solution holds the maximum amount of solute at a certain temperature, it is saturated. Any additional solute that is added will just fall to the bottom of the beaker. Solubility is the quantity of solute that dissolves in a given amount of solvent. The solubility of a solute depends on the nature of the solute and solvent and temperature. Most solids are more soluble in water (solvents) at higher temperatures. So, heating the solution can increase the amount of solute that dissolves. Solubility is expressed as the mass of solute per 100 g of water at a specific temperature. Objective In this experiment, you will identify a mystery solute by making a solubility curve using 3 concentrations of the solute. Materials Balance Mass Boat Hot Plate Thermometer 3 Test tubes Test tube rack 400 or 600 ml beaker Mystery solute PASCO temperature probe Water 10 ml graduated cylinder Stirring rod Procedure 1. One lab partner should complete steps 2-3, while the other partner begins on step 4. 2. Clean and rinse four test tubes and place them into a test tube rack. Using tape, label them: 1, 2, 3 3. Mass the correct amounts of the mystery solute to prepare the test tubes as indicated below: Please do not forget to re-zero the balance after you place the mass boat on the balance! Each test tube will also get exactly 5 mL of distilled water. Test Tube # 1 Grams of mystery Solute 3.5 mL of distilled H2O 5 2 3.0 5 3 2.5 5 4. Fill a 600 mL (or 400 mL) beaker about ¾ full of hot tap water. This will be used as a hot water bath. Heat this water bath on the hot plate. -1- 5. Place test tube #1 into the water bath and stir with a glass stirring rod until the solute is completely dissolved. Remove the tube from the water bath and place it into a wooden test tube rack. 6. Place the PASCO temperature probe (or thermometer) into test tube #1. Record the exact single temperature when you first see crystallization. NOTE: IF USING A BUNSEN BURNER, KEEP THE PROBE AND WIRE AWAY FROM THE BUNSEN BURNER! 7. Repeat steps 5 and 6 for all three test tubes. If you want to finish this lab on time, you and your partners should figure out how to divide up the work so you can have more than one test tube in the hot water bath at one time. Only leave each test tube in the water bath long enough to dissolve the entire solid. Leaving it in longer means it will take longer to cool down and crystallize. 8. You need to remove the tape from every test tube and scrub each test tube out with a soapy brush. You also need to scrub out the hard water ring from the beaker by scrubbing it with a soapy nylon sponge. Wet wipe the stir rod, thermometer, and Pasco temp. probe. Table 1 – Test tube # 1 2 3 4 A grams solute 5 mL H2O 3.5 3.0 2.5 1.85 Crystallization temp. (ºC) B grams solute 100 mL H2O 23ºC 37g Calculations 1. Convert the Column A masses to mass/100 mL (Column B) 2. Generate an XY Scatter Graph in Excel of g of mystery substance/100mL water (y axis) versus temperature (x axis). 3. Construct a solubility curve by connecting the plotted points on your graph. 4. Identify the mystery solute by matching your graph to the graph on the next page of this lab. MYSTERY SUBSTANCE: __________________________ -2- Conclusion and Questions 1. According to your graph, how does the solubility of mystery solute change as the temperature rises? 2. Using your graph, how much mystery substance must be added to 100mL (g) of water to make a saturated solution at 55 ºC? 3. Using the graph on the bottom of this page, how much mystery substance must be added to 100mL (g) of water to make a saturated solution at 55 ºC? 4. Compare your answers to #2 and #3 above and explain. 5. On your graph, label the areas that represent: saturated, unsaturated, and supersaturated Use the solubility curve provided below to determine the answers to the following questions: 6. How many grams of solute are required to saturate 100 g of water in each of the following solutions? a. KCl at 80ºC b. KClO3 at 90ºC c. NaNO3 at 10ºC 7. What is each of the solutions below: saturated, unsaturated or supersaturated? (All of the solutes are mixed with 100 g of water). a. 30 g of KCl at 10ºC b. 80 g of KNO3 at 60ºC c. 80 g of NH4Cl at 80ºC 8. At what temperature are the following solutes equally soluble in 100 g of water? a. NaNO3 and KNO3 b. NH4Cl and HCl 9. Which solute is least affected by the temperature changes? 10. Which three solutes show a decrease in solubility with increasing temperature? Requirements for Lab Report (submit as Google Doc) Your name & lab partner's name Data: Table 1 – show your calculations below the table Data Analysis: Excel Graph Conclusion Questions 1-10 -3-