XPSassign10a
advertisement

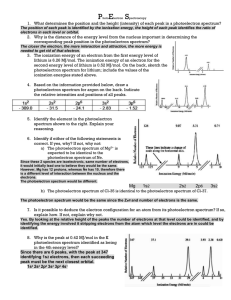
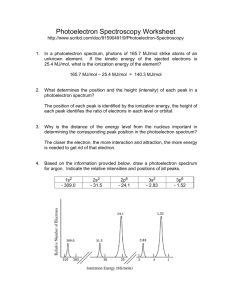
AP Chemistry Assignment: Photoelectron Spectroscopy Photoelectron Spectroscopy (PES) or X-ray Photoelectron Spectroscopy (XPS) is an experimental technique that ejects electrons from an atom or molecule and measures their energies. You will see a PowerPoint presentation on Photoelectron Spectroscopy and discuss it in class; you should take notes during this presentation. The PowerPoint slides from this presentation are posted on my web-page. In order to dot his assignment, you should first review the PowerPoint presentation and your class notes. First, write the complete electron configurations for the following elements: H, He, Li, C, O, Ca, and Sc. Second, go to the following website: www.chem.arizona.edu/chemt/Flash/photoelectron.html You may have to adjust your browser settings or update your Flash player in order to view and use this web app. Third, the photoelectron app will allow you to generate the X-ray photoelectron (XPS) spectrum for some elements. Generate the spectra as indicated below and answer the questions. These questions will be collected and graded (they should be typed): 1. Select the dual spectra mode by clicking on “Dual”. This will allow you to compare the XPS spectra for two elements. 2. Click on “Assign 1” then click on H (on the periodic table). The spectrum for hydrogen will appear on the left. Click on “Assign 2” then click on He (on the periodic table). The spectrum of helium will appear on the right. a. The numbers above the peaks in the spectra represent the relative number of electrons detected. b. The number below the peaks in the spectra represent the Binding Energy (BE) of those electrons in units of electron volts (eV). c. Q1: Which energy level do hydrogen’s and helium’s electrons occupy? d. Q2: Why do helium’s electrons have a higher binding energy than Hydrogen’s if they are in the same energy level? 3. Change Spectrum 2 to lithium by clicking on Assign 2, then clicking on Li. The hydrogen spectrum should remain on the left and the lithium spectrum should now appear on the right. a. Q3: Explain the differences in the two spectra in terms of the electron configurations of each element (H and Li). 4. Change Spectrum 1 to carbon and Spectrum 2 to oxygen. a. Q4: Why does the lowest binding energy peak change from 2 to 4 electrons? 5. Change Spectrum 1 to calcium and Spectrum 2 to scandium. a. Q5: Make a table with three columns: Orbital, Calcium, and Scandium. In the orbital column, list the orbital (one per row) in order of increasing energy (1s, 2s, NFA: APChem: W.Istone: 10/2014 b. c. d. e. f. g. 2p etc.). In the columns for Calcium and Scandium, record the number of electrons in each orbital and their binding energies. Q6: What is the peak in the Scandium spectrum at 0.63 eV due to? Q7: What is the peak in the Scandium spectrum at 0.77 eV due to? Q8: The formula for scandium chloride is ScCl3. Based upon this formula, how many valence electrons does Scandium have? Q9: In which orbital(s) is / are Scandium’s valence electrons? Q10: How do your answers for Q8 and Q9 explain the fact that transition metals have a variable number of valence electrons? Q11: Write a paragraph explaining how photoelectron spectroscopy data supports the quantum theory of the atom. NFA: APChem: W.Istone: 10/2014