PhotoElectron Spectroscopy What determines the position and the
advertisement

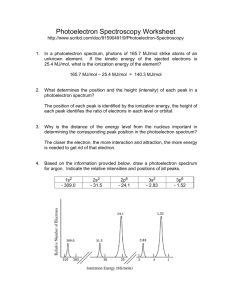
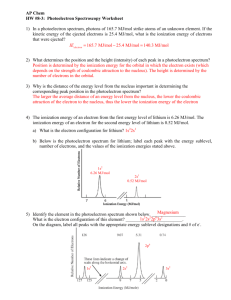
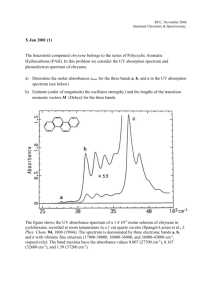
PhotoElectron Spectroscopy 1. What determines the position and the height (intensity) of each peak in a photoelectron spectrum? The position of each peak is identified by the ionization energy, the height of each peak identifies the ratio of electrons in each level or orbital. 2. Why is the distance of the energy level from the nucleus important in determining the corresponding peak position in the photoelectron spectrum? The closer the electron, the more interaction and attraction, the more energy is needed to get rid of that electron. 3. The ionization energy of an electron from the first energy level of lithium is 6.26 MJ/mol. The ionization energy of an electron for the second energy level of lithium is 0.52 MJ/mol. On the back, sketch the photoelectron spectrum for lithium; include the values of the ionization energies stated above. 4. Based on the information provided below, draw a photoelectron spectrum for argon on the back. Indicate the relative intensities and positions of all peaks. 5. Identify the element in the photoelectron spectrum shown to the right. Explain your reasoning. 6. Identify if either of the following statements is correct. If yes, why? If not, why not: a) The photoelectron spectrum of Mg2+ is expected to be identical to the photoelectron spectrum of Ne. Since these 2 species are isoelectronic, same number of electrons, it would initially lead one to believe they would be the same. However, Mg has 12 protons, whereas Ne has 10, therefore there is a different level of interaction between the nucleus and the electrons. The photoelectron spectrum would be different. Mg 1s2 2s2 2p6 3s2 b) The photoelectron spectrum of Cl-35 is identical to the photoelectron spectrum of Cl-37. The photoelectron spectrum would be the same since the Z eff and number of electrons is the same. 7. Is it possible to deduce the electron configuration for an atom from its photoelectron spectrum? If so, explain how. If not, explain why not. Yes. By looking at the relative height of the peaks the number of electrons at that level could be identified, and by identifying the energy involved it stripping electrons from the atom which level the electrons are in could be identified. 8. Why is the peak at 0.42 MJ/mol in the K photoelectron spectrum identified as being in the 4th energy level? Since there are 6 peaks, with the peak at 347 identifying 1s2 electrons, then each succeeding peak must be the next closest orbital. 1s2 2s2 2p6 3s2 3p6 4s1