National Dementia Diagnostics Laboratory Cerebrospinal Fluid
advertisement

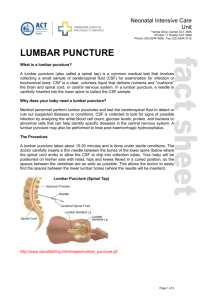
National Dementia Diagnostics Laboratory Cerebrospinal Fluid (CSF) Alzheimer Disease Screen The sample Routine diagnostic lumbar puncture can be performed any time of day. Fasting is not required. 1. A volume of CSF is first collected into standard diagnostic tubes for routine biochemical and microscopic assessments at the point of collection. A further 2-5 ml is collected directly into a polypropylene tube (and stored in the same tube for transportation. Do NOT centrifuge. Record the time of collection on the tube. Each sample is clearly labelled with a patient identifier. 2. A copy of the original doctor’s request slip, Referral laboratory request form and the specimen data sheet (download from the website) should accompany the sample. Please provide accurate billing details with accompanying documentation. 3 Freeze the specimen and store at -20oC and courier batched with other CSF specimens on dry ice within 2 months of lumbar puncture. 4. Please provide routine cell counts and biochemistry results as soon as possible to facilitate interpretation of the assay results and also avoid testing unsuitable CSF specimens. 5. Please notify our laboratory that the CSF sample is being sent. Information about the test 1. The CSF samples will be run in batches to minimise costs. Current estimates are fortnightly assays of samples. 2. Unsuitable samples: CSF samples may be deemed unsuitable for testing if they display any of the following2: very elevated protein levels (>1g/L); evidence of pleocytosis; significant blood contamination (>500 RBCs/μl); or if there is greater than 24 hours delay between lumbar puncture and receipt of the specimen and the sample has not been stored according to the sampling protocol. The referring laboratory/clinician will be contacted upon receipt of such samples and advised. 3. Fees: Currently Nil. Charging will commence in near future (An estimate is Aus$190 for testing three proteins per CSF specimen). This costing is based on batching. Testing may be possible prior to routine batching however the costing will increase. Please contact the laboratory for a quotation. Reporting Results The result will be reported by mail and/or email to the referring laboratory and requesting medical practitioner. Contact/Courier details: Dr Qiao-Xin Li, National Dementia Diagnostics Laboratory, The Florey Institute of Neuroscience and Mental Health, Kenneth Myer Building, 30 Royal Parade (corner of Genetics Lane), University of Melbourne, Parkville, VIC 3010, email: q.li@unimelb.edu.au, Phone: (03) 9035 7243, Facsimile: (03) 9035 3105.