Supplementary materials Characteristics of Korean Patients with
advertisement

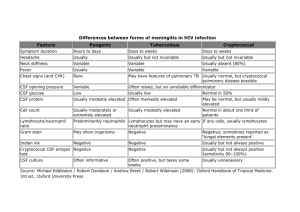
Supplementary materials Characteristics of Korean Patients with Suspected Creutzfeldt-Jakob Disease with 14-3-3 Protein in Cerebrospinal Fluid: Preliminary Study of the Korean Creutzfeldt-Jakob Disease Active Surveillance Program Running title: Active Surveillance System for Suspected Creutzfeldt-Jakob Disease in Korea Supplementary Methods CSF 14-3-3 protein assay 14-3-3 protein levels were analyzed in the CSF with Western blot. Assay protocol was accredited for standard operating procedure (SOP) in the Korean National Institute of Health. CSF was centrifuged before the Western blot. This was for the elimination of blood or other contamination, and for the exclusion of nonspecific background. The specimen was kept at 4°C before the blotting, and stored at -70°C when it should be kept more than one year. Furthermore, we have conducted whole blood test also. Genetic analysis of the prion protein gene (PRNP) mutation and that of the polymorphism at codon 129 was carried out using DNA extracted from blood specimens according to standard method. For the CSF 14-3-3 analysis, the samples were mixed with buffers and boiled for 10 min. The CSF samples of the confirmed cases were managed with the same protocol as that used with the positive controls. The samples were mounted in each lane of a Nupage 4–12% Bis-Tris gel (NOV-NP0335, Life Technologies Korea, Seoul, Korea). Novex® sharp prestained protein standards were used as lane markers. Electrophoresis was done in 120 mA for 50 minutes; the resultant gel was transferred to a polyvinylidene difluoride membrane in 70 V for 7 minutes using dry blot system (iBlot Gel Device, INV-IB1001, Life Technologies Korea, Seoul, Korea). The polyvinylidene difluoride membrane was then activated by soaking it in methanol for 5 minutes, and it was then treated with 5% skim milk for 1 hour, which was prepared by melting it in 0.1% Tween 20-containing phosphate-buffered solution (PBS)buffer (PBS-T). Thereafter, it was treated with a monoclonal antibody against N-terminus of human 14-3-3 (pan 14-3-3 antibody (H-8), SC-1675, Santa Cruz, CA, USA) at a 1:1,000 working dilution in PBS-T for 2 hours. It was sensitive for 7 isoforms of 14-3-3 proteins with beta, epsilon, eta, gamma, sigma, theta, and seta. The membrane was rinsed with PBS-T 3 times and treated with horseradish peroxide-conjugated secondary antibodies for 1 hour. It was then again rinsed with PBS-T 3 times, illuminated with an enhanced chemiluminescence reagent (Super Signal, West Pico, PIERCE, USA) in a darkroom, and exposed to x-ray film (Eastman Kodak Company, Rochester, NY, USA). The film was developed and fixed for the detection of the ~30-kDa 14-3-3 protein in the CSF. Supplementary Table. Requested numbers of CSF 14-3-3 protein assay according to study period. 2006 2010 2011 Total Study duration, months 5 14 9 28 Requested numbers of CSF 14-3-3 protein 46 108 64 218 26 53 27 106 20 55 37 112 assays Number of subjects with CSF 14-3-3 protein (+) Number of subjects with CSF 14-3-3 protein (-)