Homework Set #2
advertisement

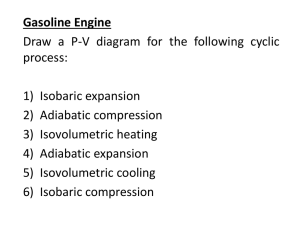
ATSC5010 Homework #2 (Sept 16) Due beginning of class Sept 23 1. In class today we discussed how a diesel engine works. In our example we assumed that during the intake phase, air entering the engine was at 20 degC. We also assumed that the diesel fuel would ignite at 500 degC at the top of the compression stroke. What is the minimum compression ratio necessary that would allow this diesel engine to work? For the engine to operate in a colder environment, would it require higher, lower, or the same compression? Why? 2. Calculate the change in specific internal energy and specific enthalpy for each of the following: a. Isothermal expansion from v = 900 to 960 cm3 g-1, at T=300K b. Isobaric heating from -10 to +10C at p=1000 hPa. 3. Beginning with our definition of enthalpy as: h = u +pv and using the ideal gas law, show that: 𝜕ℎ | =0 𝜕𝑝 𝑇 4. A sample of 50 g of dry air is initially at a pressure of 1000 mb and a temperature of 10 C. It is heated in an isobaric process during which the sample expands by 10% of its original volume. Calculate the final temperature of the air, the ‘work’ done, and the amount of heating. 5. An Ideal Gas is confined to a volume, Va in an insulated rigid container. The container has an adjoining volume Vb, initially evacuated. Assume Va and Vb are equal. Further assume that the initial pressure in Va is 1000 mb. A valve is opened and gas flows freely from Va to Vb. a. Calculate the work done by the gas in expansion. b. Calculate the change in internal energy of the gas. c. Calculate the change in entropy of the gas. d. Is this process adiabatic? e. Is this process reversible? 6. Consider two reversible processes operating on two separate systems. Both systems have the same initial sate. One process is an isothermal expansion, the other is an adiabatic expansion. Assume that at the end of the processes, the pressure of the two systems is the same. Compare the change in volume between the two systems. Sketch the two processes on a P-V diagram.