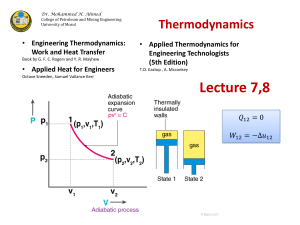
True or False questions 1. For a closed gaseous system the value of ʃpdV for the change of the gas from one given state to another is independent of the path so long as all processes are reversible. 2. The molar heat capacity at constant pressure, cp, of an ideal gas is independent of pressure. 3. The enthalpy of an ideal gas is a function of temperature only. 4. The first law of thermodynamics requires that the total energy of any system be conserved within the system. 5. The equation pvγ=constant is valid for any adiabatic process of an ideal gas. Answers: 1. False. The integral represents work which is path dependent. 2. True. For an ideal gas. 3. True. 4. False. 5. False. It is true only is the process is also reversible.