Lab chemical or physical RxN

Name__________________________________________________Period_________Date___________
Chemical or Physical Change Lab
Purpose: Use what you’ve learned about chemical and physical changes to determine if the following reactions are chemical or physical. Make sure to provide evidence for your observations.
Safety Procedure: goggles are required for the entire lab
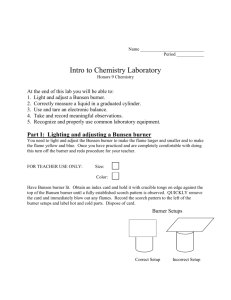
Experiment 1: Heat the unknown in a crucible
Material: Bunsen burner, flint lighter, ring stand, clay triangle, crucible, tongs, Epsom salt
Procedure: Set up your ring stand like the picture. Bring the crucible to the front and obtain a scoop of Epsom salt. Heat the salt in a crucible until you see a change take place 60 secs.
Clean up: Use tongs to remove the crucible. Dispose of the salt in the trash and let the crucible cools naturally (do not dip in water)
Was it a chemical or physical change?
_______________________________________________________
What evidence do you have to back up your guess?
__________________________________________________________
__________________________________________________________
_________________________________________________________
Experiment 2: Ignition of a metal
Material: Bunsen burner, flint lighter, tongs, magnesium ribbon
Procedure: Move the ring stand away from the Bunsen burner. Obtain magnesium from Mr. Vu. Use tongs hold on to 1 end of the magnesium and ignite it in the Bunsen burner. Let the flame die out on its own.
Clean up: Dispose of the used magnesium in the trash
Was it a chemical or physical change? _______________________________________________________
What evidence do you have to back up your guess? ______________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
Experiment 3: Dissolved or not dissolved
Material: glass beaker, acetone, spatula, Styrofoam pieces
Procedure: Acetone is flammable! make sure your Bunsen burner is off for this part. Pour 10-20 ml of acetone into the beaker, obtain Styrofoam 2 pieces, and place the Styrofoam into acetone.
Clean up: use the spatula to fish the Styrofoam pieces out and dispose in the trash can. Pour the used acetone to the “used acetone” beaker to the front.
Was it a chemical or physical change? _________________________________________________________
What evidence do you have to back up your guess? ______________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
Experiment 4: Combine two solutions
Material: test tube, glass beaker
Procedure: Obtain 2 ml of AgNO
3
solution and put in test tube, obtain 2 ml NaCl solution and put in beaker.
Add the NaCl solution into the test tube.
Clean up: Dispose of the combined solutions from the test tube in the waste beaker located inside the hood.
Rinse the test tube and beaker before putting them away.
Was it a chemical or physical change? _________________________________________________________
What evidence do you have to back up your guess? ______________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
Read each scenario. Decide whether a physical or chemical change has occurred and give evidence:
Scenario Chemical or
Physical Change?
Explanation
A student removes a loaf of bread hot from the oven. The student cuts a slice off the loaf and spreads butter on it.
Your friend decides to toast a piece of bread, but leaves it in the toaster too long. The bread is black and the kitchen if full of smoke.
You forgot to dry the bread knife when you washed it and reddish brown spots appeared on it
Chewing food to break it down into smaller particles represents a _________ change, but the changing of starch into sugars by enzymes in the digestive system represents a
___________change.
In a fireworks show, the fireworks explode giving off heat and light.
Food color is dropped into water to give it color.
A straight piece of copper wire is coiled to form a spring.
You blow dry your wet hair
Teacher’s Notes:
Materials: Bunsen burner, flint lighter, ring stand, clay triangle, crucible, tongs
Small glass beaker, spatula, test tube
Epsom salt, magnesium ribbon, acetone, Styrofoam, 0.5M AgNO3 sol., 0.5 M NaCl sol.
Waste beaker (hood), used acetone beaker, pipets (2), 10ml graduated cylinder (2)
Chemicals that are interesting to heat:
1) Magnesium sulfate heptahydrate (Epsom salts: The crystals jump around!)
2) White flame
3) Styrofoam deformed -
4) White precipitation
Scenario Explanation Chemical or
Physical Change?
Physical A student removes a loaf of bread hot from the oven. The student cuts a slice off the loaf and spreads butter on it.
No change in substances. No unexpected color change, temperature change or gas given off.
Chemical Chemical Started with bread, ended with smoke. Chemical reactions
Your friend decides to toast a piece of bread, but leaves it in the toaster too long. The bread is black and the kitchen if full of smoke.
You forgot to dry the bread knife when you washed it and reddish brown spots appeared on it
Chewing food to break it down into smaller particles represents a _________ change, but the changing of starch into sugars by enzymes in the digestive system represents a
___________change.
In a fireworks show, the fireworks explode giving off heat and light.
Food color is dropped into water to give it color.
A straight piece of copper wire is coiled to form a spring.
Chemical chemical
Physical
Physical
Spots of rust result from a chemical reaction
Physical/ chemical Chewing is physical because it just changes shape and size. Enzymes change sugars into new compounds so that is chemical
Fire/explosions/burning is always chemical
Water and food color don’t react to form something new, they just mix together
Only changes shape, you start with copper wire and end with copper wire
You blow dry your wet hair Physical Phase change from liquid water to gas water. Start with and end with water