Molecules or Ions
advertisement

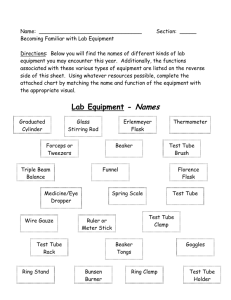
Molecules or Ions? Page 1 of 3 SAFE WORK PROCEDURE LOCATION VMC WRITTEN BY: APPROVED BY: Science Team DATE CREATED WS&H LAST REVISION June 27, 2014 New PERSONAL PROTECTION EQUIPMENT (PPE) Safety glasses or face shield must be worn at all times in work areas. Long and loose hair must be tied back Appropriate footwear must be worn. Shoe must be fully enclosed. No open toed shoes. Close fitting/protective clothing must be worn. Remove strings hanging from pullovers/sweaters. Rings and jewelry (long necklaces / bracelets, etc.) must not be worn. HAZARDS PRESENT Chemical burns Absorption of Chemicals. Inhalation of Chemicals. Ingestion of Chemicals. Slips / trips & falls Chemical hazards Burns from the Bunsen burner ADDITIONAL REQUIREMENTS APPARATUS / MATERIALS Magnifying lens 5 beakers Scoopula Stirring rod Conductivity tester Bunsen burner Test tube rack 4 test tubes Test tube holder Labels Distilled water Solid honey Epsom salts Lauric acid Washing soda Equipment orientation WHMIS training SAFE WORK PROCEDURE 1. Don all personal protective equipment including: safety glasses, & protective nitrile gloves (where required). Ensure all loose clothing is either removed or tied back. Remove any jewelry and tie back long hair. 2. Examine a small sample of each substance (water, honey, Epsom salts, lauric acid, washing soda) using a magnifying lens. Record its appearance. Note, in particular, if the edges of the sample have a characteristic shape. 3. Caution: Use the proper technique to carefully smell each compound. If you detect an odour, try to describe it in your table. 4. You must wear protective gloves for this step. Test the hardness of each substance by rubbing a small sample between your thumb and forefinger. Use words such as “soft”, “waxy”, SAFE WORK PROCEDURE Molecules or Ions? Page 2 of 3 and “brittle” to record your observations. Wash your hands thoroughly when you have completed this step, and follow your teacher’s instructions for cleaning the gloves. 5. Label each beaker with the name of one of the test substances. Pour 50 ml of distilled water into each beaker. Label one beaker “control”. 6. Predict whether the control will conduct electricity or not. Then test you prediction and record your results. 7. Use the scoopula to add a peanut-sized quantity of honey to the appropriate beaker. Stir with a stirring rod, and note whether the substance dissolves completely. 8. Test the solution for conductivity, and record your results. 9. Repeat steps 7 and 8 for each of the other substances. 10. Label each test tube with the name of one of the test substances. Use the scoopula to add a peanut-sized quantity of honey to the appropriate test tube. 11. Caution: Using the tongs, gently heat the test tube over a Bunsen burner flame. If the substance melts, record the relative melting point as “low”. If the substance does not melt, record the relative melting points as “high”. 12. Repeat steps 10 and 11 for each of the other substances. 13. After the test tubes have cooled, clean them and put them away. Wash your hands. 14. Clean up your workstation. REGULATORY REQUIREMENTS WS&H Act W210, Section 4, 5 Mb. Regulations 217/2006, Part 16, (Machines / Tools & Robots) Sections 16.1-16.18) Part 35, (WHMIS Application) Part 36, (Chemical & Biological Substances Application)