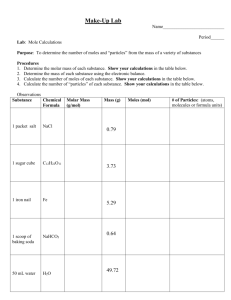
CBA Review #1 2015
advertisement
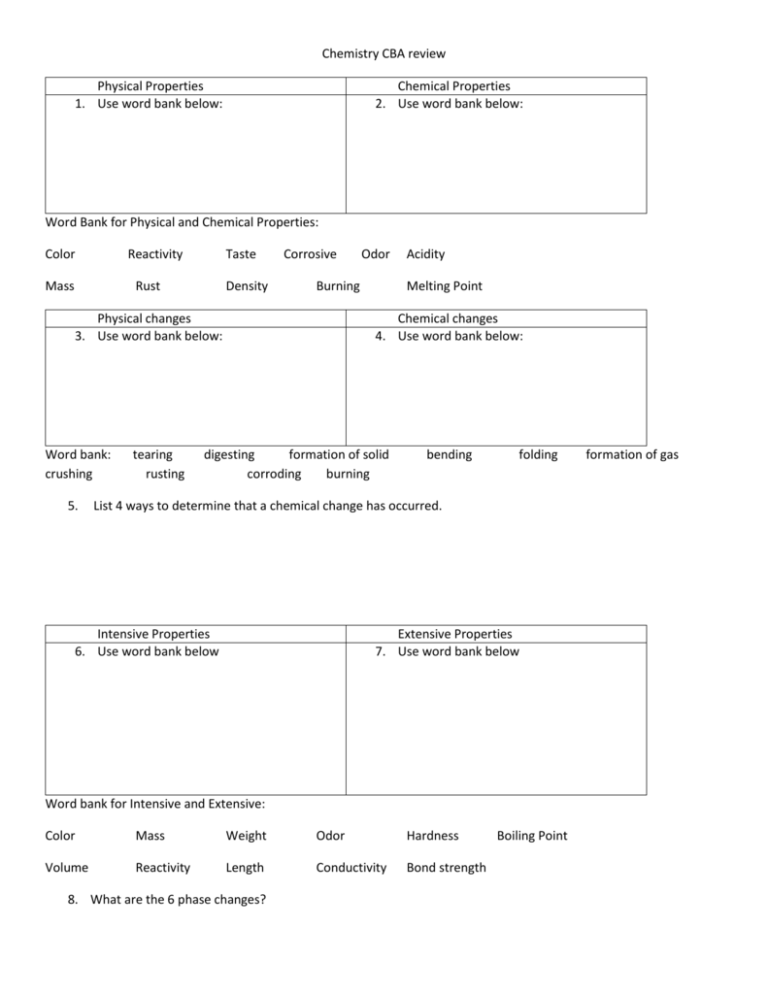
Chemistry CBA review Physical Properties 1. Use word bank below: Chemical Properties 2. Use word bank below: Word Bank for Physical and Chemical Properties: Color Reactivity Mass Taste Rust Density Corrosive Burning Physical changes 3. Use word bank below: Word bank: crushing 5. tearing rusting Odor Acidity Melting Point Chemical changes 4. Use word bank below: digesting formation of solid corroding burning bending folding List 4 ways to determine that a chemical change has occurred. Intensive Properties 6. Use word bank below Extensive Properties 7. Use word bank below Word bank for Intensive and Extensive: Color Mass Weight Odor Hardness Volume Reactivity Length Conductivity Bond strength 8. What are the 6 phase changes? Boiling Point formation of gas 9. What is a mixture? 10. What is a compound? Give examples! 11. What is an element? Give Examples! 12. How can you tell the difference between a mixture and a compound? Homogeneous Mixture 13. Definition: Heterogeneous Mixture 14. Definition: 15. Examples: Use word bank below 16. Examples: Use word bank below Word Bank for Homogeneous and Heterogeneous mixtures: Air Soil Salt solution Vinegar Kool Aid Calcium Carbonate Alcohol 17. List methods of separation for mixtures. Moles to Mass 17. What is the mass of 2.00 moles of Na? Milk Water 18. What is the mass of 2.5 moles of C? 19. What is the mass of 12.5 moles of He? Grams to Moles 20. How many moles are in 50.0 g of Ag? 21. How many moles are in 25.0 g of Ca? 22. How many moles are contained in 45.0 g of Na? Grams to Atoms 23. How many atoms are in 25 g of Ne? 24. How many atoms are in 25g of O2 ? 25. How many grams are present in a sample of carbon that contains 8.47x10 24 atoms? 26. What is the average atomic mass of Boron if it exists as 19.90% 10B (10.013 g/mol) and 80.10%11B (11.009 g/mol)? 27. What are the parts of the atom? Where are the individual parts located? 28. How are fission and fusion alike? How are fission and fusion different? 29. Describe alpha, beta and gamma particles? How can each of the particles be blocked? 30. List the symbol for the following nuclear particles: Alpha Beta gamma neutron proton 31. What is the wavelength of light in nm, that has a frequency of 6.6 x 10 14 Hz? 32. What is the frequency of red light with a wavelength of 6.90 x 10-7m? 33. Describe the relationship between wavelength and frequency. ( use the table from your notes) 34. Balance the following nuclear equations: a. 27 13 Al + b. 63 29 Cu + c. 44 20 4 2 He 2 1 30 15 P + ____________ H 2 01 n + ____________ Ca + 11 H 44 21 Sc + ____________