What`s in a Compound?
advertisement
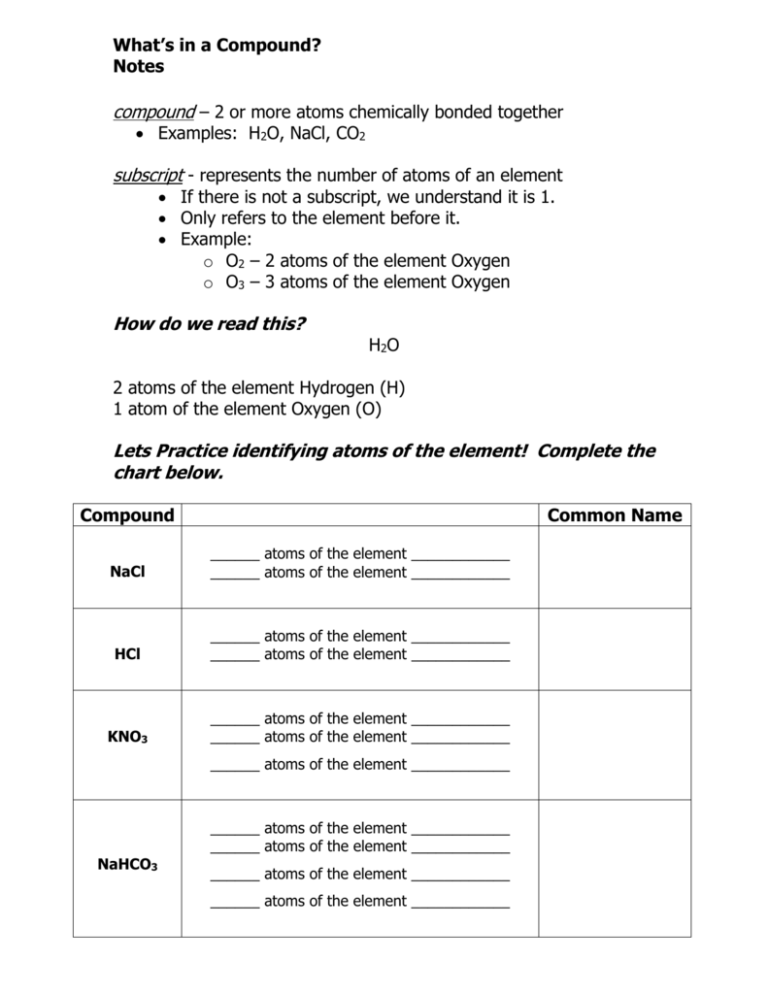
What’s in a Compound? Notes compound – 2 or more atoms chemically bonded together Examples: H2O, NaCl, CO2 subscript - represents the number of atoms of an element If there is not a subscript, we understand it is 1. Only refers to the element before it. Example: o O2 – 2 atoms of the element Oxygen o O3 – 3 atoms of the element Oxygen How do we read this? H2 O 2 atoms of the element Hydrogen (H) 1 atom of the element Oxygen (O) Lets Practice identifying atoms of the element! Complete the chart below. Compound Common Name NaCl ______ atoms of the element ____________ ______ atoms of the element ____________ HCl ______ atoms of the element ____________ ______ atoms of the element ____________ KNO3 ______ atoms of the element ____________ ______ atoms of the element ____________ ______ atoms of the element ____________ NaHCO3 ______ atoms of the element ____________ ______ atoms of the element ____________ ______ atoms of the element ____________ ______ atoms of the element ____________ Lets Practice! How many atoms in a compound with a coefficient? Coefficient – represents the number of molecules of a compound If there is not a coefficient, we understand it to be 1. Only refers the compound behind it. o Example: 3HCl – 3 molecules of the compound hydrochloric acid 2H2O – 2 molecules of the compound water The coefficient gets distributed and multiplied throughout the compound to determine how many atoms of each element. o Example: 2H2O – The 2 gets distributed to the H and the O H = 4 atoms of the element hydrogen (2x2) O = 2 atoms of the element oxygen (2x1) Let’s practice distributing! Compound How many atoms of each element? ______ atoms of the element ____________ 2CO2 Carbon Dioxide ______ atoms of the element ____________ ______ atoms of the element ____________ 2H2SO4 (Sulfuric acid) ______ atoms of the element ____________ ______ atoms of the element ____________ ______ atoms of the element ____________ 3H2O2 (Hydrogen Peroxide) ______ atoms of the element ____________ ______ atoms of the element ____________ Now let’s put it all together! Compound What is in the compound? _____ molecules of the compound _____________________ 3HCl (Hydrochloric Acid) is made up of ______ atoms of the element ____________ ______ atoms of the element ____________ _____ molecules of the compound _____________________ 2FeS2 (Fools Gold) is made up of ______ atoms of the element ____________ ______ atoms of the element ____________ _____ molecules of the compound _____________________ C6H12O6 (Glucose) is made up of ______ atoms of the element ____________ ______ atoms of the element ____________ ______ atoms of the element ____________








