Author Bio: Yik Zhang recommends that everyone keeps a journal to
advertisement

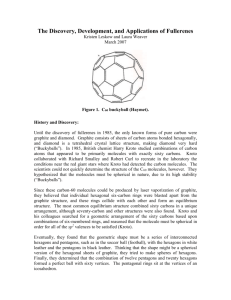
Author Bio: Yik Zhang recommends that everyone keeps a journal to be filled with thoughts, innovations, questions, and drawings of things in life. Also he begrudgingly does ChemE by being unprepared for class. Key Words: Nanotechnology, material science, fullerene, carbon, sphere, nobel, buckyball Written on: October 17th 2013 Prepared for USC Illumin Article The Curiously Awesome Buckminsterfullerene: A Spherical Introduction Abstract: Memorable in its shape and herculean in its structural integrity and strength, Carbon-60 (C60), or more affectionately called the buckminsterfullerene or buckyball, is the third allotrope of carbon after graphite and diamond (Fig. 1) [1]. While its extraordinary stability was initially suspect to highly circumstantial evidence, continual research eventually verified this coveted property and set off major investigations toward its Michael Strӧck/ Wikimedia Commons Figure 1: Eight examples of carbon allotropes.a is diamond, b is graphite, and d is C60 scientific curiosity and technological applications. Aside from its mesmerizing spherical symmetry, the buckyball has been identified as a potential candidate for use in cancer treatment and has even been theorized to explain the origin of life. Introduction and History: While nanotechnology appears to be a relatively recent field, the first application of it can be traced back to medieval Europe's beautifully stained church windows. Interestingly enough, the discovery of the C60 molecule was a noted yet unintentional peculiarity within an experiment involving the laser evaporation of graphite. Throughout the joint research experiment, researchers Harold Kroto from University of Sussex, and Robert Curl, Richard Smalley, and graduate student James Heath of Rice University noted a recurring peak associated with 720 atomic mass units that correlates to a group of sixty carbon atoms [2]. The intrigue of what structural form could possibly encompass this large group of atoms eventually drove the group towards the conclusion of soccer ball-like structure. The discovery in 1985 by the senior researchers later won them the 1996 Noble Prize in Chemistry, drawing international attention to the field of pure carbon molecules, now classified under the term fullerenes. Complementing the uniqueness of its shape is the origin of the name buckminsterfullerene where it was named after its resemblance to the architecture of geodesic domes (Fig. 2) and made famous by American architect Richard Buckminster [2]. While C60 is the most famous carbon cluster, later research Erik16/Wikimedia Commons Figure 2: Picture of Spaceship Earth at Epcot, Walt Disney World, and an example of a geodesic sphere. by Smalley and his colleagues showed that other carbon clusters like C26 and C70 that exist. Normally, such prestigious awards are not given to discoveries of single molecules, however the buckyball discovery lead not only to new, useful derivatives, but invoked within the scientific community a reinvigoration of interest regarding carbon's versatility and role in future science exploration. Properties: C60 is composed of twenty hexagons and twelve pentagons (Fig. 3), with a carbon atom at each polygon's vertex and a sp2 hybridized bond and 60 π bonds spread throughout the molecule [2]. The mathematics behind the geodesic resemblance finds that Wikimedia Commons Figure 3: A depiction of a basic buckyball structure. Shown are 20 hexagons and 12 pentagons. this truncated icosahedral cage structure grants stability at high temperature and pressure [3] due to its distribution of molecular strain [4]. It is at the pentagon locations that the molecule is most susceptible to chemical attacks, a point which will later be revisited [5]. One property relating to size is its shared property with quantum dots, another nanomaterial. Both materials act as the role of the acceptor and are photosensitive or reactive to the properties of light in that it is capable of absorbing certain wavelengths of light from neighboring molecules. Aqueous solutions of pure C60 are purple in color as it is the result of the individual molecules absorbing wavelengths associated with green and emitting both red and blue wavelengths [6]. When compressed to less than 70% of its initial volume, the solid form of the molecule will exhibit physical properties akin to the hardness of a diamond though will quickly regain its original volume when the pressure is released [5]. Lastly, there is a theoretical limit to the stable formation for these molecules. All evennumbered clusters ranging from C3 to C600 are hollow fullerenes though below C32, the structure is unable to maintain its stability [5]. Various applications can benefit from these properties and will be detailed in the following section. Applications: Going back to the process of photoinduced electron transfer, one such application proposes that C60 and a semiconductor polymer known as MEH-PPV can be separately spun on top of one another to create a heterojunction device [7]. This device enables a large photoresponse due to the transfer of electrons from the donor, the polymer, to the acceptor, C60. In general, both the mechanical and electrical engineering fields can appreciate this process as to create new materials that have the electronic and optical properties of semiconductors while maintaining the mechanical properties and advantages associated with polymers [7]. The most useful aspect of buckyball is its various derivatives that involve changing the molecule's surface chemistry and constituent groups. A modification by insertion, replacement, or attachment of atoms or constituent groups alters the properties of the molecule yet will maintain the familiar spherical shape. One application involves the buckyball as a transport molecule. As the sixty carbon atoms are arranged and spaced out in a manner that leaves the interior empty, this vacuous area is capable of sheltering and isolating atoms Wikimedia Commons Figure 4: A general example of an endohedral metallofullerene. The red sphere is the metal atom. or small molecules. These alterations, specifically for metal atoms, define a specific kind of buckyball, the endohedral metallofullerene (Fig. 4). It was also discovered that irradiation with intense laser light causes the carbon skeleton to shrink in size. The inwards collapse will eventually cease when the skeleton fits just comfortably around the metal atom. This forms a molecule that protects its contents within a sturdy carbon frame and becomes easier to interact with as opposed to attempts to directly transport material like radioactive isotopes or highly sensitive biomaterial used to treat cancer cells. EERE/ Wikimedia Commons Figure 5: Example of refueling a hydrogen vehicle. Perhaps the most exciting one with regards to energy storage is the buckyball's ability to have transition metal atoms be bound to its surface. Hydrogen as it currently stands is viewed as a clean energy alternative to fossil fuels though massive adoption in automotive technology has yet to occur due to hazards involving high pressure hydrogen environments that could lead to hazardous combustions. However, through the buckyball, suitable and efficient absorbents for high density, room temperature, ambient pressure storage of hydrogen can be created [8]. Furthermore, the optical and photophysical applications have generated a noticeable amount of research and attention. One researcher has demonstrated that films of polyvinylcarbazole doped with fullerenes are an excellent photoconductor while another found that fullerene films treated in plasma have contributed to greater nucleation and synthesis of diamond films on silicon surfaces [1]. Future: As with most immerging technologies, the future of the utility and ubiquity of the buckyball is intrinsically tied to its cost and appeal to researchers and industry alike. Yet as researcher Arthur F. Hedbard, the individual responsible for leading the discovery of superconductivity on the buckyball field states, "There remain an uncountable number of intercalation possibilities not only with respect to the pristine fullerene framework but also with respect to modified fullerene frameworks consisting of fullerenes with encapsulated metals atoms or replaced carbon atoms" [1].Theoretically, buckyballs can be related to any molecule and there lies its greatest value. A greater adaptation of the buckyball’s transport capabilities within society inspires what can only be described as virtually boundless progress in medicine, science, and technology. Works Cited: [1] A.F. Hebard, "Buckminsterfullerene", Annu. Rev. Mater. Sci, vol. 23, pp. 159-191, 1993 [2] H.W. Kroto et al, "C60: Buckminsterfullerene", Chem. Rev., vol. 91, no. 6, pp. 1213-1235, May, 1991. [3] A. Karton et al, "Evaluation of the heats of formation of corannulene and C60 by means of high-level theoretical procedures", J. of Phys. Chem. A, vol. 117, no. 8, pp. 1834, 2013. [4] S. Tomita et al, "Stability of Buckminsterfullerene, C60", Chemical Physics Letters, vol. 383, pp. 120-125, Nov, 2003. [5] Randall Frost, "Buckminsterfullerene", in Gale Encyclopedia of Science, 4th ed., Ed K Lee Lerner and Brenda Wilmoth Lerner Detroit, Gale Virtual Ref. Library, 2008, pp. 680-681. [6] M.S. Dresselhaus et al, "Science of fullerenes and carbon nanotubes", Academic Press, pp. 437, 1996. [7] N.S. Sarieiftei et al, "Photoinduced Electron Transfer from a Conducting Polymer to Buckminsterfullerene", Science: New Series, vol. 258, no. 5087, pp. 1474-1476, Nov, 1992. [8] Yufeng Zhao et al, "Hydrogen Storage in Novel Organometallic Buckyballs", Physical Review Letters, vol. 94, no. 15, pp.155504(1-4), Apr, 2005.