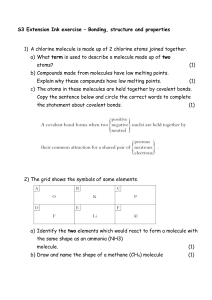
File molecules elements vs compounds pre
advertisement
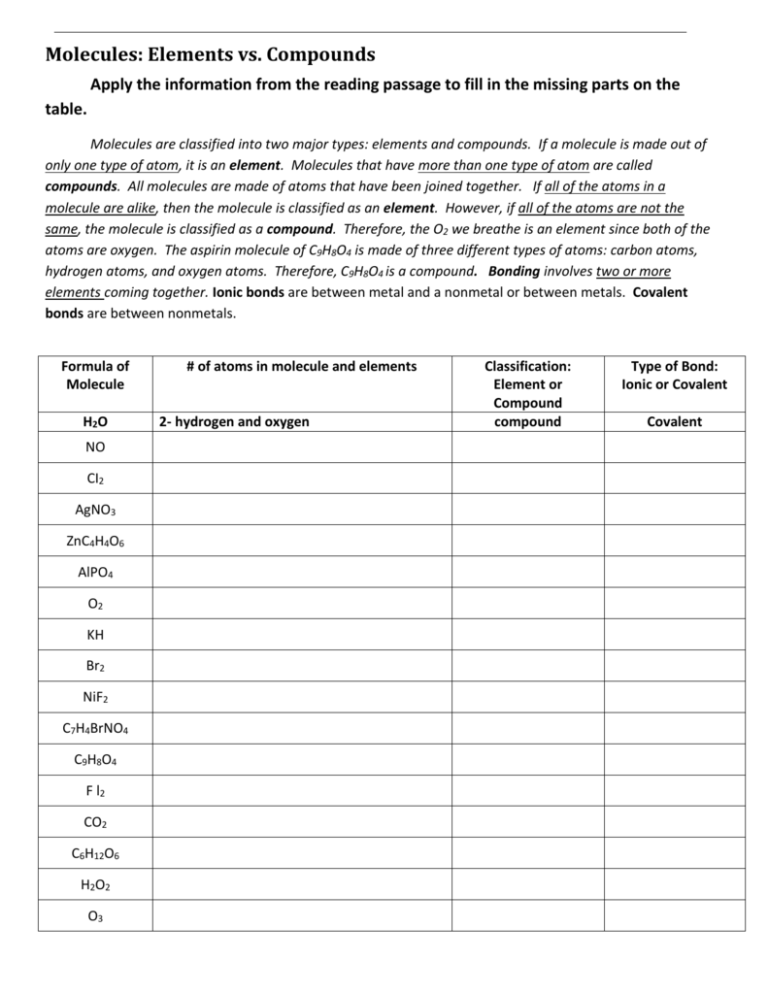
Molecules: Elements vs. Compounds Apply the information from the reading passage to fill in the missing parts on the table. Molecules are classified into two major types: elements and compounds. If a molecule is made out of only one type of atom, it is an element. Molecules that have more than one type of atom are called compounds. All molecules are made of atoms that have been joined together. If all of the atoms in a molecule are alike, then the molecule is classified as an element. However, if all of the atoms are not the same, the molecule is classified as a compound. Therefore, the O2 we breathe is an element since both of the atoms are oxygen. The aspirin molecule of C9H8O4 is made of three different types of atoms: carbon atoms, hydrogen atoms, and oxygen atoms. Therefore, C9H8O4 is a compound. Bonding involves two or more elements coming together. Ionic bonds are between metal and a nonmetal or between metals. Covalent bonds are between nonmetals. Formula of Molecule H2O NO CI2 AgNO3 ZnC4H4O6 AlPO4 O2 KH Br2 NiF2 C7H4BrNO4 C9H8O4 F l2 CO2 C6H12O6 H2O2 O3 # of atoms in molecule and elements 2- hydrogen and oxygen Classification: Element or Compound compound Type of Bond: Ionic or Covalent Covalent