TLC & HPLC Analysis of Nitroanilines Lab Assignment
advertisement

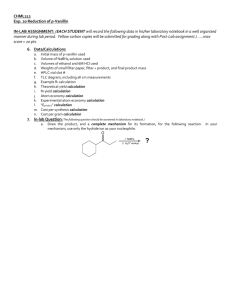
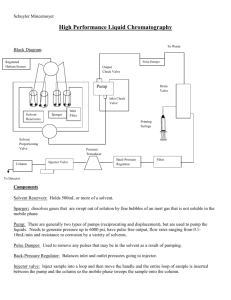
CHML211 Exp. 4 TLC and HPLC Analysis of Nitroanilines IN-LAB ASSIGNMENT: (EACH STUDENT will record the following data in his/her laboratory notebook in a well organized manner during lab period. Yellow carbon copies will be submitted for grading along with Post-Lab assignment.) …..max score = 20 pts. 6. Data/Calculations (The following information should be recorded directly into laboratory notebook.) a. b. c. Names of TLC developing solvent systems (3) Sketch of all TLC plates. Each spot detected should be labeled with the appropriate cm measurement, as well as the cm measurement for the solvent front on each plate All TLC Rf calculations 7. In-lab Questions (The following questions should be answered in laboratory notebook.) a. Complete the following table in your laboratory notebook: TLC HPLC Stationary phase Mobile phase Indication of Purity Indication of Identity Polar analyte Nonpolar analyte Effect of increasing solvent system polarity… b. List the interm0lecular forces present in each of the compounds below. Which compound would have a greater affinity for the stationary phase in liquid chromatography? How would this affect the TLC R f value? How would this affect the HPLC retention time? Which compound would have a greater affinity for the mobile phase in liquid chromatography? How would this affect the TLC R f value? How would this affect the HPLC retention time of this compound? O OH C benzoic acid naphthalene CHML211 Exp. 4 TLC and HPLC Analysis of Nitroanilines POST-LAB ASSIGNMENT: (EACH LAB GROUP will submit one copy of a typewritten, paragraph style report addressing all of the points listed below. Must be written using PAST TENSE, PASSIVE VOICE. ) …..max score = 50 pts. 8. Experimental (Write 1-2 paragraphs including all of the following. Do NOT present a bulleted outline.) Describe the process used to prepare the TLC plates, including: o How the plate itself was prepared o How the compounds were applied to the plate o Which compounds were applied to the plate Describe the process used to prepare the TLC developing chamber, including: o Which solvents were added to the chambers o The volume of each solvent used Describe how the plates were developed, and how the compounds were identified Describe the process of analyzing the HPLC results, including: o How were the HPLC results presented? o How were the compounds in each HPLC chromatogram identified? 9. Results (Copy and paste the completed tables into your document.) Table 4.1 TLC Results (This table can be completed by hand, once copy/pasted into document!) Rf values in Solvent System 1 80:20 hexane/ ethyl acetate ANALYTE TLC Rf Data Rf values in Solvent System 2 50:50 hexane/ ethyl acetate Rf values in Solvent System 3 100% ethyl acetate o-nitroaniline p-nitroaniline mixture TLC Diagrams (Show measurements for solvent front and all spots in cm) X o X mix X p X o X mix X p X o X mix Table 4.2 HPLC Results (This table can be completed by hand or computer, once copy/pasted into document!) ANALYTE o-nitroaniline p-nitroaniline STANDARD Rt (min) HPLC Rt Data SAMPLE SAMPLE Rt (min) Rt (min) Solvent System Solvent System 1 2 SAMPLE Rt (min) Solvent System 3 X p 10. Discussion (Write 1-2 paragraphs including the following.) What effect did increasing the TLC developing solvent polarity have on the R f values of the individual compounds? How would this affect your ability to separate these compounds from one another? Support your conclusion using actual numerical TLC Rf data from Table 4.1. What effect did increasing the HPLC eluting solvent polarity have on the Rt values of the individual compounds? How would this affect your ability to separate these compounds from one another? Support your conclusion using actual numerical HPLC Rt data from Table 4.2. If your compounds were contaminated with trace amounts of ethyl acetate, hexane, or water, would this contaminant be detected and appear as a peak using HPLC analysis? Briefly explain considering the type of detector used in HPLC analysis. Include a short comment addressing what could be done differently to improve the experimental results, if repeated.