January 5 2016
advertisement

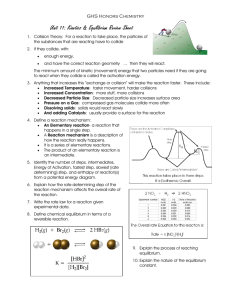
Le Châtelier and His Principle Warm-Up (1) A mixture of H2 and I2 is allowed to react at 448oC. When equilibrium is established, the concentrations of the participants are found to be [H2] = 0.46 M, [I2] = 0.39 M, and [HI] = 3.0 M. Calculate the value of the Keq at 448oC. H2(g) + I2(g) <=====> 2 HI(g) (2) Calculate the equilibrium constant, Keq, for the following reaction at 25 °C, if [NO] = 0.106 M, [O2] = 0.122 M and [NO2]eq = 0.129 M. 2 NO (g) + O2 (g) 2 NO2 (g) Warm-Up Answers (1) (2) Keq = 12.1 Homework Answers EQUILIBRIUM: [SO3]2 (1) Keq = ---------------------------------[SO2]3 [O2] 1 (3) Keq = ------------------------------------[H2]2 [O2] (5) Keq = [CO2] (2) n/a = all liquids [CH4] [H2O] (4) Keq = --------------------------------------------------[H2]3 [CO] Homework Answers (6) 0.133 (7) Keq < 1 thus reactants favored (8) 4.01 (9) 0.50 (10) 1.41 M (11) 0.599 (12) 9.81 X 10 -3 (0.00981) Solutions/Acid & Bases RETEST Grades posted in Power School ! NO Retest Tutoring – BUT mandatory test review after school on Today. You MUST come in and look over your previous test before taking the retest. You MUST plan to RETEST on Wednesday ATTENDANCE RECOVERY Last day for attendance recovery is THURSDAY, January 7, 2016! All forms must be submitted by Friday, January 8, 2106! If you have any questions about your attendance…please see me ASAP!! MAKE-UP WORK ALL make-up work must be submitted by this Friday, January 8, 2016 The Week Ahead…. Day Mon / Tues Wed What We’ll Be Doing Unit 11 – Equilibrium Unit 11 QUIZ (Grade) Reference Table Scavenger Hunt (Grade) Exam Review Day 1 Thurs Exam Review Packet – Grade Sheet #1 Take/Discuss Released Test (Short Version) Exam Review Day 2 Fri Exam Review Packet – Grade Sheet #2 Cumulative Review Demo – Post-Test (Grade) Exam Review Day 3 Mon Exam Review Packet – Grade Sheet #3 Take/Discuss Released Test (Long Version) Exam Review Day 4 Tues Exam Review Packet – Grade Sheet #4 Cumulative Review Activity Final Exam Reminders Le Châtelier’s Principle If a stress is applied to a system at equilibrium, the system shifts to reduce the stress. The 3 stresses of Chemistry 1. Concentration 2. Temperature PRODUCTS 3. Pressure REACTANTS Concentration Increasing the concentration of one of the reactants or products causes a shift toward the other side. Decreasing the concentration of one of the reactants or products causes a shift toward the same side. Temperature Treat heat as a product for exothermic Treat heat as a reactant for endothermic Refer back to rules for concentration Pressure Note-only applies if there is an unequal amount of gases on each side Increasing the pressure will cause a shift toward the side with the lower number of moles of gas. Decreasing the pressure will cause a shift toward the side with the higher number of moles of gas. Learning Team Challenge N2 (g) + H2 (g) ⇆ NH3 (g) Would the equilibrium shift toward the reactants OR products if…… Hydrogen was removed? Ammonia was added? Hydrogen was added? Pressure was increased? 12.6 J + H2 (g) + I2 (g) 2HI (g) How would the equilibrium shift when the following stresses are applied to the system? (1) Add hydrogen iodide (2) Increase the temperature (3) Decrease the pressure (4) Remove Iodine UNIVERSITY OF EQUILIBRIUM http://www.education.uoit.ca/lordec/ID_LORDEC/c hemistry_lechateliers/index.html CAN YOU GRADUATE WITH HONORS? Hawkins’ Wiki Page – January 5 – Click on the Link for University of Equilibrium - Start with Admissions Requirements - Complete Years 1 – 4 - Take Final Exam – show Hawkins’ Score You try! C2H2 (g) + H2O (g) ⇆ CH3CHO (g) The above reaction is endothermic, predict the shift in the equilibrium (1) You increase CH3CHO (2) You decrease C2H2 (3) You increase H2O (4) You decrease the temperature Goals You should now be able to… Predict what side of the reaction will be favored given a stressor Exit Ticket For the equilibrium system (1) Write the equation for the calculating the equilibrium constant How will the above reaction shift if: (2) More chlorine is added? (3) Pressure is increased (note: all reactants and products are gases) BONUS: Keq = 35. If the concentrations of PCl5 and PCl3 are 0.025M and 0.68M respectively, what is the concentration of the Cl2? HOMEWORK Worksheet Equilibrium: #13 Le Chatelier’s Principle: ALL (1 – 5) – omit 2 D UNIT 11 QUIZ TOMORROW !! BRING YOUR ORGANIZED NOTEBOOK TOMORROW TO BEGIN EXAM REVIEW!!