Salinity age of earth calculation activity
advertisement

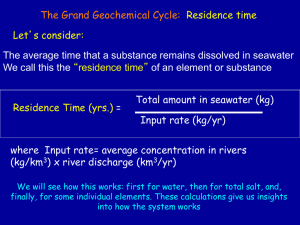
PART 2: Calculating the age of the earth using salinity data Purpose: Students will attempt to calculate the age of the earth using salinity data in order to gain familiarity with a 19th century method of calculating the age of the earth and to identify and explain the shortcomings of the method. Introduction: Edmond Halley (1656 – 1742), of Halley’s comet fame, hypothesized that seawater became salty with the infusion of salts dissolved from the ground. He proposed the dissolved salts were carried down rivers to the sea and gradually accumulated, raising the salinity of seawater over time. In 1899, using Halley’s hypothesis, an Irish geologist, John Joly (1857 – 1933), proposed a method for calculating the age of the oceans and perhaps the earth as well. Joly reasoned that if the salinity of the oceans was known, along with the rate at which salt was being added by rivers each year, the calculation could be done. Activity: See the sodium data and sample calculation below. The calculation uses Joly’s line of reasoning to calculate the age of the earth using the data. Discuss the calculation with your group. Later, you will use the same method of calculation using magnesium rather than sodium. 1) 2) 3) 4) The volume of the world’s oceans is 1,370,000,000 km3 Sodium makes up approximately 1% of seawater by volume The world’s rivers empty a volume of 30,000 km3/yr into the oceans Rivers contain between 3 to 11 ppm (parts per million) Sodium by volume Sample calculation: 1) Calculate the total volume of sodium in the world’s oceans. This would be 1% of the total volume of water. So, (.01)(1,370,000,000 km3) = 13,700,000 km3 salt in oceans. 2) Calculate how much sodium enters the oceans from the rivers annually. Since there is a range of concentrations, calculate both high and low. 3ppm: (.000003)(30,000km3) = .09km3 salt entering oceans from rivers 11ppm: (.000011)(30,000km3) = .33km3 salt entering oceans from rivers 3) Divide total volume of sodium in ocean by annual volume input. Calculate for high and low river input to get a range. 13,700,000 km3 salt/.09km3 salt/year = ~ 152 million years 13,700,000 km3 salt/.033km3 salt/year = ~ 41 million years See the data for Magnesium below. Use the sample calculation as a guide and perform the simplified version of Joly’s calculation using Magnesium. 5) 6) 7) 8) The volume of the world’s oceans is 1,370,000,000 km3 Magnesium makes up approximately .14% of seawater by volume The world’s rivers empty a volume of 30,000 km3/yr into the oceans Rivers contain approximately 4 ppm (parts per million) Magnesium by volume Calculation: Age estimate using magnesium ___________________________ Age estimate using sodium ____________________________ Summarizing Questions. 1. Compare the values you got in your calculations with Na and with Mg. How close were they? What inferences can you make regarding which ion is removed from rivers and oceans at a faster rate? Brainstorm reasons for any differences you noted. 2. How close are the calculated values to your prior understanding of the age of the earth? 3. Identify any assumptions you can think of that this method of calculating the age of the earth relies on for validity. Also indicate whether that assumption is correct or incorrect, and explain your thinking in the last column. Assumption Correct/Incorrect Salt stays put once in the ocean Incorrect Reasoning Plate tectonics causes seawater to pass through the ocean crust causing salt to be removed from the water Scientist ideas: Joly’s attempt to calculate the age of the earth was innovative at the time, however his reasoning is based on some faulty assumptions leading to a significant underestimate of the age of the earth. A better way to describe the values obtained through this calculation is as “residence time,” or the time that salt stays in oceans before being removed through one of many processes. Sodium and Magnesium are removed through different processes and thus at different rates, accounting for the varying values obtained using each ion. The main assumption that Joly made that led to the underestimate of the earth’s age is his assumption that salt stays in the oceans once deposited. There are several processes that remove salt from the oceans including the process of plate tectonics that causes salt removal from seawater as it passes through the ocean’s crust. Salt is also removed from deposition of salt in sediments on the sea floor, and through transfer onto land from sea spray. The method also assumes that the influx of salt in to the oceans has been constant over time. Weather rates have been known to change drastically throughout geologic time, so this assumption is likely false. Magnesium is removed from seawater by various processes that differ from sodium’s removal. For example, magnesium reacts with calcium carbonate in coral islands, through this process, the magnesium becomes incorporated into the coral and thus is removed from the ocean water. The rate at which magnesium is removed is higher, than the removal of sodium, resulting in a larger underestimate of the age of the earth using this calculation method with magnesium as the ion under consideration. Discuss your responses to #1-3 and the scientists’ ideas above with your group. Would you change any of your answers? Explain your thinking.