Solubility Graph WS
advertisement

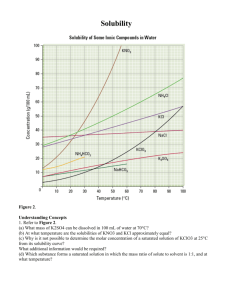
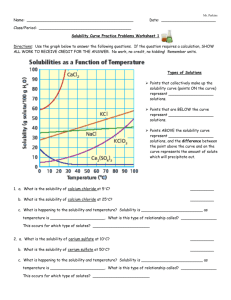
Name______________________________________ Date_________________________ Pd________ Solubility Graph Use the graph to answer the following questions. The lines represent the predicted solubility of each substance. 1. Why do the temperatures on the graph only go from 0˚C to 100˚C?____________ _____________________________________________________________________ _____________________________________________________________________ 2. Which substance is most soluble at 60˚C? ________________________________ 3. Which two substance have the same solubility at 80˚C?_____________________ _____________________________________________________________________ 4. 5. 6. 7. Which substance’s solubility changes most from 0˚C to 100˚C? _______________ Which substance’s solubility changes least from 0˚C to 100˚C? _______________ What is the solubility of potassium nitrate at 90˚C?_________________________ At what temperature does potassium iodide have a solubility of 150g/ 100cm3?_____ 8. You have a solution of sodium nitrate containing 140 g at 65˚C. Is the solution saturated or unsaturated? Explain how you know this.______________________ _____________________________________________________________________ _____________________________________________________________________ 9. You have a solution of potassium chlorate containing 4g at 65˚C. How many additional grams of solute must be added to it, to make the solution saturated?_________________________________________________________ 10. In the space below sketch the graph for solubility of a gas dissolved in water as the temperature increases. Solubility in g/100cm3 water Temperature in Degrees Celsius